Behavior of the Surviving Population of Listeria monocytogenes and Salmonella Typhimurium Biofilms Following a Direct Helium-Based Cold Atmospheric Plasma Treatment
- PMID: 35401458
- PMCID: PMC8988229
- DOI: 10.3389/fmicb.2022.831434
Behavior of the Surviving Population of Listeria monocytogenes and Salmonella Typhimurium Biofilms Following a Direct Helium-Based Cold Atmospheric Plasma Treatment
Abstract
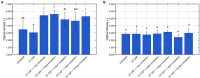
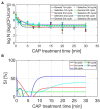
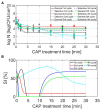
Although the Cold Atmospheric Plasma (CAP) technology proved promising for inactivation of biofilms present on abiotic food contact surfaces, more research is required to examine the behavior of the CAP surviving biofilm-associated cells. It was therefore examined whether (i) CAP treated (Listeria monocytogenes and Salmonella Typhimurium) biofilm-associated cells were able to further colonize the already established biofilms during a subsequent incubation period and (ii) isolates of the surviving population became less susceptible toward CAP when the number of biofilm development-CAP treatment cycles increased. For this purpose, a direct treatment was applied using a helium-based Dielectric Barrier Discharge electrode configuration. Results indicated that the surviving population was able to further colonize the already established biofilms, since the cell density of the CAP treated + incubated biofilms equaled the initial density of the untreated biofilms. For the L. monocytogenes biofilms, also the total biomass proved to further increase, which might result in an even further increased resistance. The susceptibility of the biofilm-associated cells proved to be influenced by the specific number of CAP treatment cycles, which might potentially result in an overestimation of the CAP treatment efficacy and, consequently, an increased risk of food contamination.
Keywords: Listeria monocytogenes; Salmonella Typhimurium; biofilms; cold atmospheric plasma; regrowth; resistance and susceptibility; surviving population.
Copyright © 2022 Govaert, Smet, Acquah, Walsh and Van Impe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Dual-Species Model Biofilm Consisting of Listeria monocytogenes and Salmonella Typhimurium: Development and Inactivation With Cold Atmospheric Plasma (CAP).Front Microbiol. 2019 Nov 7;10:2524. doi: 10.3389/fmicb.2019.02524. eCollection 2019. Front Microbiol. 2019. PMID: 31787943 Free PMC article.
-
Combined Effect of Cold Atmospheric Plasma and Hydrogen Peroxide Treatment on Mature Listeria monocytogenes and Salmonella Typhimurium Biofilms.Front Microbiol. 2019 Nov 20;10:2674. doi: 10.3389/fmicb.2019.02674. eCollection 2019. Front Microbiol. 2019. PMID: 31824459 Free PMC article.
-
Inactivation of L. monocytogenes and S. typhimurium Biofilms by Means of an Air-Based Cold Atmospheric Plasma (CAP) System.Foods. 2020 Feb 6;9(2):157. doi: 10.3390/foods9020157. Foods. 2020. PMID: 32041294 Free PMC article.
-
Fighting Mixed-Species Microbial Biofilms With Cold Atmospheric Plasma.Front Microbiol. 2020 May 20;11:1000. doi: 10.3389/fmicb.2020.01000. eCollection 2020. Front Microbiol. 2020. PMID: 32508796 Free PMC article. Review.
-
An ecological perspective of Listeria monocytogenes biofilms in food processing facilities.Crit Rev Food Sci Nutr. 2013;53(8):801-17. doi: 10.1080/10408398.2011.561378. Crit Rev Food Sci Nutr. 2013. PMID: 23768144 Review.
References
-
- Ahmed M. N., Porse A., Sommer M. O. A., Høiby N., Ciofu O. (2018). Evolution of antibiotic resistance in biofilm and planktonic Pseudomonas aeruginosa populations exposed to subinhibitory levels of ciprofloxacin. Antimicrob. Agents Chemother. 62, e00320–e00418. doi: 10.1128/AAC.00320-18, PMID: - DOI - PMC - PubMed
-
- Banu M. S., Sasikala P., Dhanapal A., Kavitha V., Yazhini G., Rajamani L. (2012). Cold plasma as a novel food processing technology. IJETED 4, 803–818.
LinkOut - more resources
Full Text Sources
Miscellaneous

