The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition
- PMID: 35401470
- PMCID: PMC8988990
- DOI: 10.3389/fmicb.2022.807955
The Chemical Relationship Among Beta-Lactam Antibiotics and Potential Impacts on Reactivity and Decomposition
Abstract
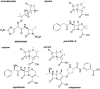
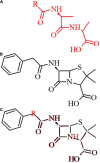
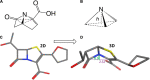
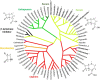
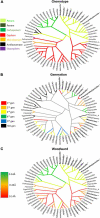
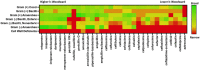
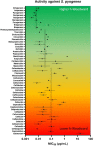
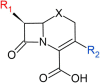
Beta-lactam antibiotics remain one of the most commonly prescribed drug classes, but they are limited by their propensity to cause hypersensitivity reactions (e.g., from allergy to anaphylaxis) as well as by the emergence of bacteria with a myriad of resistance mechanisms such as β-lactamases. While development efforts continue to focus on overcoming resistance, there are ongoing concerns regarding cross-contamination of β-lactams during manufacturing and compounding of these drugs. Additionally, there is a need to reduce levels of drugs such as β-lactam antibiotics in waste-water to mitigate the risk of environmental exposure. To help address future development of effective remediation chemistries and processes, it is desired to better understand the structural relationship among the most common β-lactams. This study includes the creation of a class-wide structural ordering of the entire β-lactam series, including both United States Food and Drug Association (US-FDA)-approved drugs and experimental therapies. The result is a structural relational map: the "Lactamome," which positions each substance according to architecture and chemical end-group. We utilized a novel method to compare the structural relationships of β-lactam antibiotics among the radial cladogram and describe the positioning with respect to efficacy, resistance to hydrolysis, reported hypersensitivity, and Woodward height. The resulting classification scheme may help with the development of broad-spectrum treatments that reduce the risk of occupational exposure and negative environmental impacts, assist practitioners with avoiding adverse patient reactions, and help direct future drug research.
Keywords: antibiotic allergy (including penicillin and cephalosporin β-lactams); antimicrobial activity; chemical informatics; deactivation; decomposition; hydrolysis; lactam antibiotics.
Copyright © 2022 Turner, Muraoka, Bedenbaugh, Childress, Pernot, Wiencek and Peterson.
Conflict of interest statement
This study received funding from Contec, Inc. The funder Contec, Inc. had the following involvement with the study: LP and MW helped conceive of the analysis, aided in analysis, co-wrote the manuscript, helped with graphical design, and aided in revisions. LP and MW were employed by Contec, Inc. MB was employed by Intramed Plus. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Evaluation and Management of Penicillin Allergy: A Review.JAMA. 2019 Jan 15;321(2):188-199. doi: 10.1001/jama.2018.19283. JAMA. 2019. PMID: 30644987 Review.
-
Allergy to beta-lactam antibiotics in children.Pediatrics. 1999 Oct;104(4):e45. doi: 10.1542/peds.104.4.e45. Pediatrics. 1999. PMID: 10506270
-
β-lactam antibiotics: An overview from a medicinal chemistry perspective.Eur J Med Chem. 2020 Dec 15;208:112829. doi: 10.1016/j.ejmech.2020.112829. Epub 2020 Sep 16. Eur J Med Chem. 2020. PMID: 33002736 Review.
-
Cephalosporins: A Focus on Side Chains and β-Lactam Cross-Reactivity.Pharmacy (Basel). 2019 Jul 29;7(3):103. doi: 10.3390/pharmacy7030103. Pharmacy (Basel). 2019. PMID: 31362351 Free PMC article. Review.
-
Broad-Spectrum Inhibitors against Class A, B, and C Type β-Lactamases to Block the Hydrolysis against Antibiotics: Kinetics and Structural Characterization.Microbiol Spectr. 2022 Oct 26;10(5):e0045022. doi: 10.1128/spectrum.00450-22. Epub 2022 Sep 7. Microbiol Spectr. 2022. PMID: 36069578 Free PMC article.
Cited by
-
Antibiotics: From Mechanism of Action to Resistance and Beyond.Indian J Microbiol. 2024 Sep;64(3):821-845. doi: 10.1007/s12088-024-01285-8. Epub 2024 Apr 29. Indian J Microbiol. 2024. PMID: 39282166 Review.
-
The Prevalence of Antimicrobial Resistance Genes in the Environments of Small Ruminant Farms from Central Portugal.Antibiotics (Basel). 2025 Jun 4;14(6):576. doi: 10.3390/antibiotics14060576. Antibiotics (Basel). 2025. PMID: 40558166 Free PMC article.
-
Precious Cargo: The Role of Polymeric Nanoparticles in the Delivery of Covalent Drugs.Molecules. 2024 Oct 19;29(20):4949. doi: 10.3390/molecules29204949. Molecules. 2024. PMID: 39459317 Free PMC article. Review.
-
Covalent penicillin-protein conjugates elicit anti-drug antibodies that are clonally and functionally restricted.Nat Commun. 2024 Aug 10;15(1):6851. doi: 10.1038/s41467-024-51138-7. Nat Commun. 2024. PMID: 39127707 Free PMC article.
-
Novel synergistic interactions between monolaurin, a mono-acyl glycerol and β lactam antibiotics against Staphylococcus aureus: an in vitro study.BMC Infect Dis. 2024 Apr 8;24(1):379. doi: 10.1186/s12879-024-09261-9. BMC Infect Dis. 2024. PMID: 38584271 Free PMC article.
References
-
- Adams C. W. (1963). Multiple factors in the pathogenesis of atherosclerosis. Guys Hosp. Rep. 112 222–253. - PubMed
-
- Aldridge S., Parascandola J., Sturchio J. L. American Chemical Society, and Royal Society of Chemistry (1999). The Discovery and Development of Penicillin 1928-1945. Washington, DC: American Chemical Society.
Publication types
LinkOut - more resources
Full Text Sources

