Core Fucosylation Regulates the Function of Pre-BCR, BCR and IgG in Humoral Immunity
- PMID: 35401499
- PMCID: PMC8990897
- DOI: 10.3389/fimmu.2022.844427
Core Fucosylation Regulates the Function of Pre-BCR, BCR and IgG in Humoral Immunity
Abstract
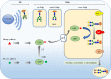
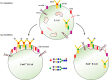
Most of the membrane molecules involved in immune response are glycosylated. N-glycans linked to asparagine (Asn) of immune molecules contribute to the protein conformation, surface expression, stability, and antigenicity. Core fucosylation catalyzed by core fucosyltransferase (FUT8) is the most common post-translational modification. Core fucosylation is essential for evoking a proper immune response, which this review aims to communicate. First, FUT8 deficiency suppressed the interaction between μHC and λ5 during pre-BCR assembly is given. Second, we described the effects of core fucosylation in B cell signal transduction via BCR. Third, we investigated the role of core fucosylation in the interaction between helper T (TH) cells and B cells. Finally, we showed the role of FUT8 on the biological function of IgG. In this review, we discussed recent insights into the sites where core fucosylation is critical for humoral immune responses.
Keywords: BCR; IgG; core fucosylation; humoral immune response; pre-B cell.
Copyright © 2022 Sun, Li, Wang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

