Specific Anti-SARS-CoV-2 Humoral and Cellular Immune Responses After Booster Dose of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine: Integrated Study of Adaptive Immune System Components
- PMID: 35401503
- PMCID: PMC8987231
- DOI: 10.3389/fimmu.2022.856657
Specific Anti-SARS-CoV-2 Humoral and Cellular Immune Responses After Booster Dose of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine: Integrated Study of Adaptive Immune System Components
Abstract
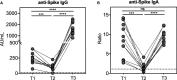
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), is modifying human activity all over the world with significant health and economic burden. The advent of the SARS-CoV-2 pandemic prompted the scientific community to learn the virus dynamics concerning transmissibility, epidemiology, and usefulness of vaccines in fighting emerging health hazards. Pieces of evidence suggest that the first and second doses of mRNA vaccines induce a significant antibody response in vaccinated subjects or patients who recovered from SARS-CoV-2 infection, demonstrating the importance of the previously formed memory. The aim of this work has been to investigate the effects of BNT162b2 Pfizer-BioNTech mRNA-based vaccine booster dose in a cohort of 11 uninfected immunocompetent (ICs), evaluating the humoral and cellular responses, with more carefulness on memory B and T cells. Our findings underscore the potential benefit of the third dose of mRNA vaccine on the lifespan of memory B and T cells, suggesting that booster doses could increase protection against SARS-CoV-2 infection.
Keywords: B cells; BNT162b2; SARS-CoV-2; T cells; booster dose; mRNA vaccine.
Copyright © 2022 Busà, Sorrentino, Russelli, Amico, Miceli, Miele, Di Bella, Timoneri, Gallo, Zito, Di Carlo, Conaldi and Bulati.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Chivu-Economescu M, Bleotu C, Grancea C, Chiriac D, Botezatu A, Iancu IV, et al. Kinetics and Persistence of Cellular and Humoral Immune Responses to SARS-CoV-2 Vaccine in Healthcare Workers With or Without Prior COVID-19. J Cell Mol Med (2022) 26(4):1293–305. doi: 10.1111/jcmm.17186 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

