PLX5622 Reduces Disease Severity in Lethal CNS Infection by Off-Target Inhibition of Peripheral Inflammatory Monocyte Production
- PMID: 35401512
- PMCID: PMC8990748
- DOI: 10.3389/fimmu.2022.851556
PLX5622 Reduces Disease Severity in Lethal CNS Infection by Off-Target Inhibition of Peripheral Inflammatory Monocyte Production
Abstract
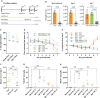
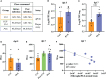
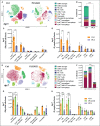
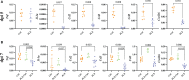
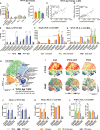
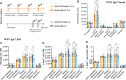
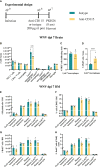
PLX5622 is a CSF-1R inhibitor and microglia-depleting reagent, widely used to investigate the biology of this central nervous system (CNS)-resident myeloid population, but the indirect or off-target effects of this agent remain largely unexplored. In a murine model of severe neuroinflammation induced by West Nile virus encephalitis (WNE), we showed PLX5622 efficiently depleted both microglia and a sub-population of border-associated macrophages in the CNS. However, PLX5622 also significantly depleted mature Ly6Chi monocytes in the bone marrow (BM), inhibiting their proliferation and lethal recruitment into the infected brain, reducing neuroinflammation and clinical disease scores. Notably, in addition, BM dendritic cell subsets, plasmacytoid DC and classical DC, were depleted differentially in infected and uninfected mice. Confirming its protective effect in WNE, cessation of PLX5622 treatment exacerbated disease scores and was associated with robust repopulation of microglia, rebound BM monopoiesis and markedly increased inflammatory monocyte infiltration into the CNS. Monoclonal anti-CSF-1R antibody blockade late in WNE also impeded BM monocyte proliferation and recruitment to the brain, suggesting that the protective effect of PLX5622 is via the inhibition of CSF-1R, rather than other kinase targets. Importantly, BrdU incorporation in PLX5622-treated mice, suggest remaining microglia proliferate independently of CSF-1 in WNE. Our study uncovers significantly broader effects of PLX5622 on the myeloid lineage beyond microglia depletion, advising caution in the interpretation of PLX5622 data as microglia-specific. However, this work also strikingly demonstrates the unexpected therapeutic potential of this molecule in CNS viral infection, as well as other monocyte-mediated diseases.
Keywords: CNS infection; CSF-1R antagonism; West Nile virus-induced encephalitis; microglia; microglia depletion; monocyte-derived cells; monocyte-mediated inflammation; neuroinflammation.
Copyright © 2022 Spiteri, Ni, Ling, Macia, Campbell, Hofer and King.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Clodronate is not protective in lethal viral encephalitis despite substantially reducing inflammatory monocyte infiltration in the CNS.Front Immunol. 2023 Jul 20;14:1203561. doi: 10.3389/fimmu.2023.1203561. eCollection 2023. Front Immunol. 2023. PMID: 37545511 Free PMC article.
-
Temporal tracking of microglial and monocyte single-cell transcriptomics in lethal flavivirus infection.Acta Neuropathol Commun. 2023 Apr 4;11(1):60. doi: 10.1186/s40478-023-01547-4. Acta Neuropathol Commun. 2023. PMID: 37016414 Free PMC article.
-
High-parameter cytometry unmasks microglial cell spatio-temporal response kinetics in severe neuroinflammatory disease.J Neuroinflammation. 2021 Jul 26;18(1):166. doi: 10.1186/s12974-021-02214-y. J Neuroinflammation. 2021. PMID: 34311763 Free PMC article.
-
Microglial Depletion, a New Tool in Neuroinflammatory Disorders: Comparison of Pharmacological Inhibitors of the CSF-1R.Glia. 2025 Apr;73(4):686-700. doi: 10.1002/glia.24664. Epub 2024 Dec 24. Glia. 2025. PMID: 39719687 Free PMC article. Review.
-
Inflammatory monocytes and the pathogenesis of viral encephalitis.J Neuroinflammation. 2012 Dec 17;9:270. doi: 10.1186/1742-2094-9-270. J Neuroinflammation. 2012. PMID: 23244217 Free PMC article. Review.
Cited by
-
Distinguished Functions of Microglia in the Two Stages of Oxygen-Induced Retinopathy: A Novel Target in the Treatment of Ischemic Retinopathy.Life (Basel). 2022 Oct 21;12(10):1676. doi: 10.3390/life12101676. Life (Basel). 2022. PMID: 36295111 Free PMC article.
-
Clodronate is not protective in lethal viral encephalitis despite substantially reducing inflammatory monocyte infiltration in the CNS.Front Immunol. 2023 Jul 20;14:1203561. doi: 10.3389/fimmu.2023.1203561. eCollection 2023. Front Immunol. 2023. PMID: 37545511 Free PMC article.
-
CNS resident macrophages enhance dysfunctional angiogenesis and circulating monocytes infiltration in brain arteriovenous malformation.J Cereb Blood Flow Metab. 2024 Jun;44(6):925-937. doi: 10.1177/0271678X241236008. Epub 2024 Feb 28. J Cereb Blood Flow Metab. 2024. PMID: 38415628 Free PMC article.
-
Inhibition of Bruton's tyrosine kinase restricts neuroinflammation following intracerebral hemorrhage.Theranostics. 2025 Jan 1;15(2):494-508. doi: 10.7150/thno.101024. eCollection 2025. Theranostics. 2025. PMID: 39744694 Free PMC article.
-
Microglia are not protective against cryptococcal meningitis.Nat Commun. 2023 Nov 8;14(1):7202. doi: 10.1038/s41467-023-43061-0. Nat Commun. 2023. PMID: 37938547 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

