Negative Immune Checkpoint Protein, VISTA, Regulates the CD4+ Treg Population During Sepsis Progression to Promote Acute Sepsis Recovery and Survival
- PMID: 35401514
- PMCID: PMC8988198
- DOI: 10.3389/fimmu.2022.861670
Negative Immune Checkpoint Protein, VISTA, Regulates the CD4+ Treg Population During Sepsis Progression to Promote Acute Sepsis Recovery and Survival
Abstract
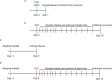
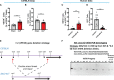
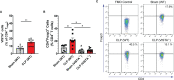
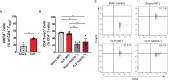
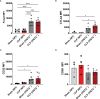
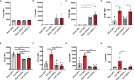
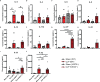
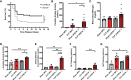
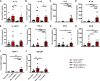
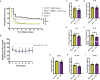
Sepsis is a systemic immune response to infection that is responsible for ~35% of in-hospital deaths and over 24 billion dollars in annual treatment costs. Strategic targeting of non-redundant negative immune checkpoint protein pathways can cater therapeutics to the individual septic patient and improve prognosis. B7-CD28 superfamily member V-domain Immunoglobulin Suppressor of T cell Activation (VISTA) is an ideal candidate for strategic targeting in sepsis. We hypothesized that immune checkpoint regulator, VISTA, controls T-regulatory cells (Treg), in response to septic challenge, thus playing a protective role/reducing septic morbidity/mortality. Further, we investigated if changes in morbidity/mortality are due to a Treg-mediated effect during the acute response to septic challenge. To test this, we used the cecal ligation and puncture model as a proxy for polymicrobial sepsis and assessed the phenotype of CD4+ Tregs in VISTA-gene deficient (VISTA-/-) and wild-type mice. We also measured changes in survival, soluble indices of tissue injury, and circulating cytokines in the VISTA-/- and wild-type mice. We found that in wild-type mice, CD4+ Tregs exhibit a significant upregulation of VISTA which correlates with higher Treg abundance in the spleen and small intestine following septic insult. However, VISTA-/- mice have reduced Treg abundance in these compartments met with a higher expression of Foxp3, CTLA4, and CD25 compared to wild-type mice. VISTA-/- mice also have a significant survival deficit, higher levels of soluble indicators of liver injury (i.e., ALT, AST, bilirubin), and increased circulating proinflammatory cytokines (i.e., IL-6, IL-10, TNFα, IL-17F, IL-23, and MCP-1) following septic challenge. To elucidate the role of Tregs in VISTA-/- sepsis mortality, we adoptively transferred VISTA-expressing Tregs into VISTA-/- mice. This adoptive transfer rescued VISTA-/- survival to wild-type levels. Taken together, we propose a protective Treg-mediated role for VISTA by which inflammation-induced tissue injury is suppressed and improves survival in early-stage murine sepsis. Thus, enhancing VISTA expression or adoptively transferring VISTA+ Tregs in early-stage sepsis may provide a novel therapeutic approach to ameliorate inflammation-induced death.
Keywords: CD25; CTLA4; Foxp3; Vista; cytokines; liver injury; regulatory T cells; sepsis.
Copyright © 2022 Gray, Biron-Girard, Wakeley, Chung, Chen, Quiles-Ramirez, Tolbert and Ayala.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
VISTA nonredundantly regulates proliferation and CD69low γδ T cell accumulation in the intestine in murine sepsis.J Leukoc Biol. 2024 May 29;115(6):1005-1019. doi: 10.1093/jleuko/qiad149. J Leukoc Biol. 2024. PMID: 38035776 Free PMC article.
-
Neutralization of interleukin-10 or transforming growth factor-β decreases the percentages of CD4+ CD25+ Foxp3+ regulatory T cells in septic mice, thereby leading to an improved survival.Surgery. 2012 Feb;151(2):313-22. doi: 10.1016/j.surg.2011.07.019. Epub 2011 Oct 6. Surgery. 2012. PMID: 21982068
-
Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis.J Immunol. 2006 Dec 1;177(11):7943-9. doi: 10.4049/jimmunol.177.11.7943. J Immunol. 2006. PMID: 17114466
-
Inhibitory Immune Checkpoint Molecule Expression in Clinical Sepsis Studies: A Systematic Review.Crit Care Med. 2020 Sep;48(9):1365-1374. doi: 10.1097/CCM.0000000000004496. Crit Care Med. 2020. PMID: 32706554 Free PMC article.
-
Immune Checkpoints: Novel Therapeutic Targets to Attenuate Sepsis-Induced Immunosuppression.Front Immunol. 2021 Feb 3;11:624272. doi: 10.3389/fimmu.2020.624272. eCollection 2020. Front Immunol. 2021. PMID: 33613563 Free PMC article. Review.
Cited by
-
VISTA expression and patient selection for immune-based anticancer therapy.Front Immunol. 2023 Feb 20;14:1086102. doi: 10.3389/fimmu.2023.1086102. eCollection 2023. Front Immunol. 2023. PMID: 36891296 Free PMC article. Review.
-
Lymphocyte HVEM/BTLA co-expression after critical illness demonstrates severity indiscriminate upregulation, impacting critical illness-induced immunosuppression.Front Med (Lausanne). 2023 May 25;10:1176602. doi: 10.3389/fmed.2023.1176602. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37305124 Free PMC article.
-
Immune Checkpoints Are New Therapeutic Targets in Regulating Cardio-, and Cerebro-Vascular Diseases and CD4+Foxp3+ Regulatory T Cell Immunosuppression.Int J Drug Discov Pharm. 2024 Dec;3(4):100022. doi: 10.53941/ijddp.2024.100022. Epub 2024 Nov 26. Int J Drug Discov Pharm. 2024. PMID: 39926714 Free PMC article.
-
VISTA nonredundantly regulates proliferation and CD69low γδ T cell accumulation in the intestine in murine sepsis.J Leukoc Biol. 2024 May 29;115(6):1005-1019. doi: 10.1093/jleuko/qiad149. J Leukoc Biol. 2024. PMID: 38035776 Free PMC article.
-
Immune dysregulation in sepsis: experiences, lessons and perspectives.Cell Death Discov. 2023 Dec 19;9(1):465. doi: 10.1038/s41420-023-01766-7. Cell Death Discov. 2023. PMID: 38114466 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

