Oncogenic Mutations and Tumor Microenvironment Alterations of Older Patients With Diffuse Large B-Cell Lymphoma
- PMID: 35401516
- PMCID: PMC8990904
- DOI: 10.3389/fimmu.2022.842439
Oncogenic Mutations and Tumor Microenvironment Alterations of Older Patients With Diffuse Large B-Cell Lymphoma
Abstract
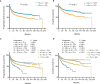
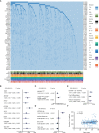
The incidence of diffuse large B-cell lymphoma (DLBCL) increases by age and older DLBCL are commonly related to poor prognosis. However, the clinical and biological features of older DLBCL patients remain to be determined. A total of 2,445 patients with newly diagnosed DLBCL were enrolled for clinical data analysis according to age at diagnosis, with tumor samples of 1,150 patients assessed by DNA sequencing and 385 patients by RNA sequencing. Older DLBCL presented advanced disease stage, elevated serum lactate dehydrogenase, poor performance status, multiple extranodal involvement, high percentage of double expressor subtype, and adverse clinical outcome. According to molecular features, age was positively correlated with the oncogenic mutations of PIM1, MYD88, BTG2, CD79B, TET2, BTG1, CREBBP, TBL1XR1, and with the MYD88-like genetic subtype. These oncogenic mutations were involved in B-cell receptor/NF-κB signaling, B-cell differentiation, and histone acetylation based on biological functions. Older DLBCL also manifested reduction in CD4+ naïve T and CD8+ naïve T cells, and also increased recruitment of exhausted T cells and macrophages, leading to immunosuppressive tumor microenvironment. Our work thus contributes to the understanding of aging-related oncogenic mutations and tumor microenvironment alterations in lymphoma progression, and may provide new insights to mechanism-based targeted therapy in DLBCL.
Keywords: B-cell receptor; aging; diffuse large B-cell lymphoma; histone acetylation; oncogenic mutations; tumor microenvironment.
Copyright © 2022 Zhu, Fu, Shi, Shi, Dong, Yi, Liu, Feng, Liu, Fang, Cheng, Wang, Tian, Xu and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Simplified algorithm for genetic subtyping in diffuse large B-cell lymphoma.Signal Transduct Target Ther. 2023 Apr 10;8(1):145. doi: 10.1038/s41392-023-01358-y. Signal Transduct Target Ther. 2023. PMID: 37032379 Free PMC article.
-
Influence of oncogenic mutations and tumor microenvironment alterations on extranodal invasion in diffuse large B-cell lymphoma.Clin Transl Med. 2020 Nov;10(7):e221. doi: 10.1002/ctm2.221. Clin Transl Med. 2020. PMID: 33252851 Free PMC article.
-
[Clinical characteristics and prognosis of primary and secondary diffuse large B-cell lymphoma of the pancreas].Zhonghua Xue Ye Xue Za Zhi. 2023 Jan 14;44(1):55-61. doi: 10.3760/cma.j.issn.0253-2727.2023.01.010. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 36987724 Free PMC article. Chinese.
-
MYD88L265P and CD79B double mutations type (MCD type) of diffuse large B-cell lymphoma: mechanism, clinical characteristics, and targeted therapy.Ther Adv Hematol. 2022 Jan 31;13:20406207211072839. doi: 10.1177/20406207211072839. eCollection 2022. Ther Adv Hematol. 2022. PMID: 35126963 Free PMC article. Review.
-
Molecular Pathogenesis of Diffuse Large B-Cell Lymphoma.J Clin Exp Hematop. 2016;56(2):71-78. doi: 10.3960/jslrt.56.71. J Clin Exp Hematop. 2016. PMID: 27980305 Free PMC article. Review.
Cited by
-
Epigenetic regulation in hematopoiesis and its implications in the targeted therapy of hematologic malignancies.Signal Transduct Target Ther. 2023 Feb 17;8(1):71. doi: 10.1038/s41392-023-01342-6. Signal Transduct Target Ther. 2023. PMID: 36797244 Free PMC article. Review.
-
Biological signatures of the International Prognostic Index in diffuse large B-cell lymphoma.Blood Adv. 2024 Apr 9;8(7):1587-1599. doi: 10.1182/bloodadvances.2023011425. Blood Adv. 2024. PMID: 38170757 Free PMC article.
-
Identification of FAT4 as a positive prognostic biomarker in DLBCL by comprehensive genomic analysis.Clin Exp Med. 2023 Oct;23(6):2675-2685. doi: 10.1007/s10238-023-01018-z. Epub 2023 Feb 22. Clin Exp Med. 2023. PMID: 36811800 Free PMC article.
-
[Clinicopathologic characteristics and prognostic analysis of testicular diffuse large B-cell lymphoma].Zhonghua Xue Ye Xue Za Zhi. 2023 Apr 14;44(4):321-327. doi: 10.3760/cma.j.issn.0253-2727.2023.04.010. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 37357002 Free PMC article. Chinese.
-
[Chinese expert consensus on the diagnosis and management of elderly patients with diffuse large B-cell lymphoma (2024)].Zhonghua Xue Ye Xue Za Zhi. 2024 Apr 14;45(4):322-329. doi: 10.3760/cma.j.cn121090-20231228-00343. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 38951058 Free PMC article. Chinese.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

