An Oral Microencapsulated Vaccine Loaded by Sodium Alginate Effectively Enhances Protection Against GCRV Infection in Grass Carp (Ctenopharyngodon idella)
- PMID: 35401526
- PMCID: PMC8987307
- DOI: 10.3389/fimmu.2022.848958
An Oral Microencapsulated Vaccine Loaded by Sodium Alginate Effectively Enhances Protection Against GCRV Infection in Grass Carp (Ctenopharyngodon idella)
Abstract
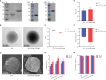
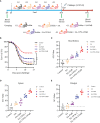
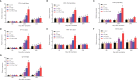
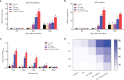
Grass carp reovirus (GCRV) is highly infectious and lethal to grass carp, causing huge economic losses to the aquaculture industry annually. Currently, vaccination is the most effective method against viral infections. Among the various vaccination methods, the oral vaccination is an ideal way in aquaculture. However, low protective efficiency is the major problem for oral vaccination owing to some reasons, such as antigen degradation and low immunogenicity. In our study, we screened the antigenic epitopes of GCRV-II and prepared an oral microencapsulated vaccine using sodium alginate (SA) as a carrier and flagellin B (FlaB) as an adjuvant, and evaluated its protective effects against GCRV-II infection in grass carp. The full length and three potential antigenic epitope regions of GCRV-II VP56 gene were expressed in Escherichia coli and purified by glutathione affinity column respectively. The optimal antigen (VP56-3) was screened by enzyme-linked immunosorbent assay (ELISA). Adjuvant FlaB was also expressed in E. coli and purified by Ni2+ affinity column. Subsequently, we prepared the oral vaccines using sodium alginate as a carrier. The vaccine (SA-VP56-3/FlaB) forms microsphere (1.24 ± 0.22 μm), examined by transmission electron microscopy, scanning electron microscopy, and dynamic light scattering assay. SA-VP56-3/FlaB vaccine has excellent stability, slow-release, and low toxicity by dynamic light scattering assay, release dynamic assay, in vivo fluorescence imaging system, hemolytic activity and cytotoxicity. Then we vaccinated grass carp orally with SA-VP56-3/FlaB and measured immune-related parameters (serum neutralizing antibody titer, serum enzyme activity (TSOD, LZM, C3), immune-related genes ((IgM, IFN1, MHC-II, CD8 in head kidney and spleen), IgZ in hindgut)). The results showed that SA-VP56-3/FlaB significantly induced strong immune responses, compared to other groups. The highest survival rate achieved in SA-VP56-3/FlaB microencapsulated vaccine (56%) in 2 weeks post GCRV challenge, while 10% for the control group. Meanwhile, the tissue virus load in survival grass carp is lowest in SA-VP56-3/FlaB group. These results indicated that SA-VP56-3/FlaB could be a candidate oral vaccine against GCRV-II infection in aquaculture.
Keywords: flagellin (FlaB); grass carp; grass carp reovirus (GCRV); oral microencapsulated vaccine; sodium alginate (SA).
Copyright © 2022 Xu, Qiao, Huo, Liao and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
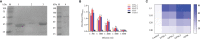

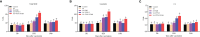
Similar articles
-
Oral Administration of Bacillus subtilis Subunit Vaccine Significantly Enhances the Immune Protection of Grass Carp against GCRV-II Infection.Viruses. 2021 Dec 24;14(1):30. doi: 10.3390/v14010030. Viruses. 2021. PMID: 35062234 Free PMC article.
-
A developed subunit vaccine based on fiber protein VP56 of grass carp reovirus providing immune protection against grass carp hemorrhagic disease.Fish Shellfish Immunol. 2019 Jul;90:12-19. doi: 10.1016/j.fsi.2019.04.055. Epub 2019 Apr 20. Fish Shellfish Immunol. 2019. PMID: 31015064
-
Display of GCRV vp7 protein on the surface of Escherichia coli and its immunoprotective effects in grass carp (Ctenopharyngodon idella).Fish Shellfish Immunol. 2018 Jan;72:199-209. doi: 10.1016/j.fsi.2017.10.060. Epub 2017 Nov 7. Fish Shellfish Immunol. 2018. PMID: 29102630
-
Insights into the antiviral immunity against grass carp (Ctenopharyngodon idella) reovirus (GCRV) in grass carp.J Immunol Res. 2015;2015:670437. doi: 10.1155/2015/670437. Epub 2015 Feb 9. J Immunol Res. 2015. PMID: 25759845 Free PMC article. Review.
-
Structural comparison and expression function analysis of BF/C2 in Ctenopharyngodon idella and Squaliobarbus curriculus.Fish Shellfish Immunol. 2023 Nov;142:109154. doi: 10.1016/j.fsi.2023.109154. Epub 2023 Oct 10. Fish Shellfish Immunol. 2023. PMID: 37821003 Review.
Cited by
-
Breaking barriers: Smart vaccine platforms for cancer immunomodulation.Cancer Commun (Lond). 2025 May;45(5):529-571. doi: 10.1002/cac2.70002. Epub 2025 Feb 3. Cancer Commun (Lond). 2025. PMID: 39901621 Free PMC article. Review.
-
Comparative Transcriptomic Analysis Revealed Potential Differential Mechanisms of Grass Carp Reovirus Pathogenicity.Int J Mol Sci. 2023 Oct 24;24(21):15501. doi: 10.3390/ijms242115501. Int J Mol Sci. 2023. PMID: 37958486 Free PMC article.
-
Gut mucosal immune responses and protective efficacy of oral yeast Cyprinid herpesvirus 2 (CyHV-2) vaccine in Carassius auratus gibelio.Front Immunol. 2022 Jul 29;13:932722. doi: 10.3389/fimmu.2022.932722. eCollection 2022. Front Immunol. 2022. PMID: 35967417 Free PMC article.
-
Rapid and sensitive detection of pathogenic Elizabethkingia miricola in black spotted frog by RPA-LFD and fluorescent probe-based RPA.Fish Shellfish Immunol Rep. 2022 Jun 24;3:100059. doi: 10.1016/j.fsirep.2022.100059. eCollection 2022 Dec. Fish Shellfish Immunol Rep. 2022. PMID: 36419595 Free PMC article.
-
Enhancing neutralizing antibodies against receptor binding domain of SARS-CoV-2 by a safe natural adjuvant system.Virus Res. 2023 Mar;326:199047. doi: 10.1016/j.virusres.2023.199047. Epub 2023 Jan 21. Virus Res. 2023. PMID: 36693449 Free PMC article.
References
-
- Wang Z, Huo X, Zhang Y, Gao Y, Su J. Carboxymethyl Chitosan Nanoparticles Loaded With Bioactive Protein CiCXCL20a Effectively Prevent Bacterial Disease in Grass Carp (Ctenopharyngodon Idella). Aquaculture (2022) 549:737745. doi: 10.1016/j.aquaculture.2021.737745 - DOI
-
- Attoui H, Fang Q, Jaafar FM, Cantaloube JF, Biagini P, de Micco P, et al. . Common Evolutionary Origin of Aquareoviruses and Orthoreoviruses Revealed by Genome Characterization of Golden Shiner Reovirus, Grass Carp Reovirus, Striped Bass Reovirus and Golden Ide Reovirus (Genus Aquareovirus, family Reoviridae). J Gen Virol (2002) 83:1941–51. doi: 10.1099/0022-1317-83-8-1941 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

