Preclinical Studies on Convalescent Human Immune Plasma-Derived Exosome: Omics and Antiviral Properties to SARS-CoV-2
- PMID: 35401544
- PMCID: PMC8987587
- DOI: 10.3389/fimmu.2022.824378
Preclinical Studies on Convalescent Human Immune Plasma-Derived Exosome: Omics and Antiviral Properties to SARS-CoV-2
Abstract
The scale of the COVID-19 pandemic forced urgent measures for the development of new therapeutics. One of these strategies is the use of convalescent plasma (CP) as a conventional source for passive immunity. Recently, there has been interest in CP-derived exosomes. In this report, we present a structural, biochemical, and biological characterization of our proprietary product, convalescent human immune plasma-derived exosome (ChipEXO), following the guidelines set forth by the Turkish Ministry of Health and the Turkish Red Crescent, the Good Manufacturing Practice, the International Society for Extracellular Vesicles, and the Gene Ontology Consortium. The data support the safety and efficacy of this product against SARS-CoV-2 infections in preclinical models.
Keywords: COVID-19; SARS-CoV-2; convalescence plasma; exosome; extracellular vehicles (EVs); viral treatment.
Copyright © 2022 Taşlı, Gönen, Kırbaş, Gökdemir, Bozkurt, Bayrakcı, Sağraç, Taşkan, Demir, Ekimci Gürcan, Bayındır Bilgiç, Bayrak, Yetişkin, Kaplan, Pavel, Dinç, Serhatlı, Çakırca, Eken, Aslan, Yay, Karakukcu, Unal, Gül, Basaran, Ozkul, Şahin, Jones, Tekin, Özdarendeli and Cetin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

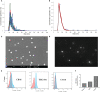
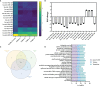
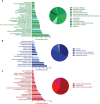
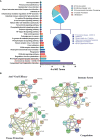

Similar articles
-
SARS-CoV-2 Neutralization in Convalescent Plasma and Commercial Lots of Plasma-Derived Immunoglobulin.BioDrugs. 2022 Jan;36(1):41-53. doi: 10.1007/s40259-021-00511-9. Epub 2021 Nov 29. BioDrugs. 2022. PMID: 34843105 Free PMC article.
-
A novel nano therapeutic using convalescent plasma derived exosomal (CPExo) for COVID-19: A combined hyperactive immune modulation and diagnostics.Chem Biol Interact. 2021 Aug 1;344:109497. doi: 10.1016/j.cbi.2021.109497. Epub 2021 May 13. Chem Biol Interact. 2021. PMID: 33991505 Free PMC article.
-
Emergence of ancient convalescent plasma (CP) therapy: To manage COVID-19 pandemic.Transfus Clin Biol. 2021 Feb;28(1):123-127. doi: 10.1016/j.tracli.2020.11.004. Epub 2020 Dec 5. Transfus Clin Biol. 2021. PMID: 33285298 Free PMC article.
-
COVID-19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: Do the exosomes in convalescent plasma antagonize the weak immune antibodies?J Extracell Vesicles. 2020 Oct;10(1):e12004. doi: 10.1002/jev2.12004. Epub 2020 Nov 14. J Extracell Vesicles. 2020. PMID: 33304473 Free PMC article. Review.
-
Convalescent Plasma Therapy for COVID-19: Lessons from SARS-CoV, MERS-CoV, and H1N1 Infection.Acta Med Indones. 2021 Jan;53(1):86-95. Acta Med Indones. 2021. PMID: 33818411 Review.
Cited by
-
Pulmonary delivery of favipiravir inhalation solution for COVID-19 treatment: in vitro characterization, stability, in vitro cytotoxicity, and antiviral activity using real time cell analysis.Drug Deliv. 2022 Dec;29(1):2846-2854. doi: 10.1080/10717544.2022.2118398. Drug Deliv. 2022. PMID: 36062490 Free PMC article.
-
Meet changes with constancy: Defence, antagonism, recovery, and immunity roles of extracellular vesicles in confronting SARS-CoV-2.J Extracell Vesicles. 2022 Dec;11(12):e12288. doi: 10.1002/jev2.12288. J Extracell Vesicles. 2022. PMID: 36450704 Free PMC article. Review.
-
CoVSense: Ultrasensitive Nucleocapsid Antigen Immunosensor for Rapid Clinical Detection of Wildtype and Variant SARS-CoV-2.Adv Sci (Weinh). 2023 May;10(15):e2206615. doi: 10.1002/advs.202206615. Epub 2023 Mar 30. Adv Sci (Weinh). 2023. PMID: 36995043 Free PMC article.
-
Chewable tablet with herbal extracts and propolis arrests Wuhan and Omicron variants of SARS-CoV-2 virus.J Funct Foods. 2023 Jun;105:105544. doi: 10.1016/j.jff.2023.105544. Epub 2023 Apr 19. J Funct Foods. 2023. PMID: 37155488 Free PMC article.
-
Extracellular vesicles in COVID-19 convalescence can regulate T cell metabolism and function.iScience. 2023 Jul 4;26(8):107280. doi: 10.1016/j.isci.2023.107280. eCollection 2023 Aug 18. iScience. 2023. PMID: 37520724 Free PMC article.
References
-
- Marciniuk DD, Schraufnagel DE. The Global Impact of Respiratory Disease. Eur Respir Soc (2017).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

