ACE2 Serum Levels as Predictor of Infectability and Outcome in COVID-19
- PMID: 35401548
- PMCID: PMC8986157
- DOI: 10.3389/fimmu.2022.836516
ACE2 Serum Levels as Predictor of Infectability and Outcome in COVID-19
Abstract
Background: COVID-19 can generate a broad spectrum of severity and symptoms. Many studies analysed the determinants of severity but not among some types of symptoms. More importantly, very few studies analysed patients highly exposed to the virus that nonetheless remain uninfected.
Methods: We analysed serum levels of ACE2, Angiotensin II and anti-Spike antibodies in 2 different cohorts at high risk of viral exposure, highly exposed but uninfected subjects, either high risk health care workers or persons cohabiting with infected close relatives and seropositive patients with symptoms. We tested the ability of the sera of these subjects to neutralize lentivirus pseudotyped with the Spike-protein.
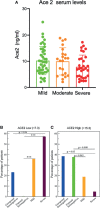
Results: We found that the serum levels of ACE2 are significantly higher in highly exposed but uninfected subjects. Moreover, sera from this seronegative persons can neutralize SARS-CoV-2 infection in cellular assays more strongly that sera from non-exposed negative controls eventhough they do not have anti-CoV-2 IgG antibodies suggesting that high levels of ACE2 in serum may somewhat protect against an active infection without generating a conventional antibody response. Finally, we show that among patients with symptoms, ACE2 levels were significantly higher in infected patients who developed cutaneous as compared with respiratory symptoms and ACE2 was also higher in those with milder symptoms.
Conclusions: These findings suggest that soluble ACE2 could be used as a potential biomarker to predict SARS-CoV-2 infection risk and to discriminate COVID-19 disease subtypes.
Keywords: ACE2; COVID-19; antibodies; biomarker; neutralization.
Copyright © 2022 Maza, Úbeda, Delgado, Horndler, Llamas, van Santen, Alarcón, Abia, García-Bermejo, Serrano-Villar, Bastolla and Fresno.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

