Improving Assignments for Therapeutic and Prophylactic Treatment Within TB Households. A Potential for Immuno-Diagnosis?
- PMID: 35401549
- PMCID: PMC8993507
- DOI: 10.3389/fimmu.2022.801616
Improving Assignments for Therapeutic and Prophylactic Treatment Within TB Households. A Potential for Immuno-Diagnosis?
Abstract
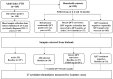
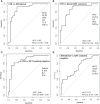
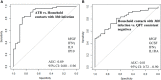
Delays in diagnosis and treatment of pulmonary tuberculosis (TB) can lead to more severe disease and increased transmission. Contact investigation among household contacts (HHCs) of TB patients is crucial to ensure optimal outcomes. In the context of a prospective cohort study in Palamaner, Southern India, this study attempted to assess the potential of 27 different soluble immune markers to accurately assign HHCs for appropriate treatment. A multiplex bead assay was applied on QuantiFERON (QFT)-nil supernatants collected from 89 HHCs grouped by longitudinal QFT status; M. tuberculosis (Mtb) infected (QFT positive at baseline and follow-up, n = 30), recent QFT converters (QFT-negative at baseline, n = 27) and converted to QFT-positivity within 6 months of exposure (at follow-up, n = 24) and QFT consistent negatives (n = 32). The 29 TB index cases represented Active TB. Active TB cases and HHCs with Mtb infection produced significantly different levels of both pro-inflammatory (IFNγ, IL17, IL8, IP10, MIP-1α, MIP1β, and VEGF) and anti-inflammatory (IL9 and IL1RA) cytokines. We identified a 4-protein signature (bFGF, IFNγ, IL9, and IP10) that correctly classified HHCs with Mtb infection vs. Active TB with a specificity of 92.6%, suggesting that this 4-protein signature has the potential to assign HHCs for either full-length TB treatment or preventive TB treatment. We further identified a 4-protein signature (bFGF, GCSF, IFNγ, and IL1RA) that differentiated HHCs with Mtb infection from QFT consistent negatives with a specificity of 62.5%, but not satisfactory to safely assign HHCs to no preventive TB treatment. QFT conversion, reflecting new Mtb infection, induced an elevated median concentration in nearly two-thirds (19/27) of the analyzed soluble markers compared to the levels measured at baseline. Validation in other studies is warranted in order to establish the potential of the immune biosignatures for optimized TB case detection and assignment to therapeutic and preventive treatment of Mtb infected individuals.
Keywords: Mtb infection; active TB; cytokine and chemokines; preventive therapy; protein signature; soluble protein markers.
Copyright © 2022 Sivakumaran, Jenum, Ritz, Vaz, Doherty and Grewal.
Conflict of interest statement
TMD is an employee of and holds shares in the GSK group of companies but participated in the current work as an independent investigator. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
[Characteristics of a diagnostic method for tuberculosis infection based on whole blood interferon-gamma assay].Kekkaku. 2006 Nov;81(11):681-6. Kekkaku. 2006. PMID: 17154047 Review. Japanese.
-
Non-IFNγ Whole Blood Cytokine Responses to Mycobacterium tuberculosis Antigens in HIV-exposed Infants.Pediatr Infect Dis J. 2021 Oct 1;40(10):922-929. doi: 10.1097/INF.0000000000003254. Pediatr Infect Dis J. 2021. PMID: 34525006 Free PMC article. Clinical Trial.
-
Longitudinal analysis of QuantiFERON-TB Gold In-Tube in children with adult household tuberculosis contact in South Africa: a prospective cohort study.PLoS One. 2011;6(10):e26787. doi: 10.1371/journal.pone.0026787. Epub 2011 Oct 31. PLoS One. 2011. PMID: 22066009 Free PMC article.
-
Biomarkers for discrimination between latent tuberculosis infection and active tuberculosis disease.J Infect. 2017 Mar;74(3):281-293. doi: 10.1016/j.jinf.2016.11.010. Epub 2016 Nov 19. J Infect. 2017. PMID: 27871809
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
Cited by
-
Host blood-based biosignatures for subclinical TB and incipient TB: A prospective study of adult TB household contacts in Southern India.Front Immunol. 2023 Jan 11;13:1051963. doi: 10.3389/fimmu.2022.1051963. eCollection 2022. Front Immunol. 2023. PMID: 36713386 Free PMC article.
References
-
- World Health Organization . Global Tuberculosis Report (2020). Available at: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-en... (Accessed on Oct 25, 2021).
-
- World Health Organization . Recommendations for Investigating Contacts of Persons With Infectious Tuberculosis in Low- and Middle-Income Countries (2012). Available at: http://www.who.int/tb/areas-of-work/laboratory/contact-investigation/en/... (Accessed on Oct 25, 2021). - PubMed
-
- World Health Organization . Systematic Screening for Active Tuberculosis: Principles and Recommendations (2013). Available at: http://www.who.int/tb/publications/tbscreening/en/.pdf (Accessed on Oct 25, 2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

