Exploring Macrophage-Dependent Wound Regeneration During Mycobacterial Infection in Zebrafish
- PMID: 35401552
- PMCID: PMC8987025
- DOI: 10.3389/fimmu.2022.838425
Exploring Macrophage-Dependent Wound Regeneration During Mycobacterial Infection in Zebrafish
Abstract
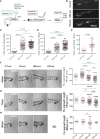
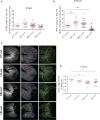
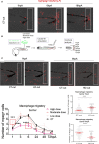
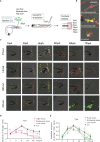
The molecular and cellular mechanisms associated with tissue degradation or regeneration in an infectious context are poorly defined. Herein, we explored the role of macrophages in orchestrating either tissue regeneration or degradation in zebrafish embryos pre-infected with the fish pathogen Mycobacterium marinum. Zebrafish were inoculated with different infectious doses of M. marinum prior to fin resection. While mild infection accelerated fin regeneration, moderate or severe infection delayed this process by reducing blastemal cell proliferation and impeding tissue morphogenesis. This was correlated with impaired macrophage recruitment at the wound of the larvae receiving high infectious doses. Macrophage activation characterized, in part, by a high expression level of tnfa was exacerbated in severely infected fish during the early phase of the regeneration process, leading to macrophage necrosis and their complete absence in the later phase. Our results demonstrate how a mycobacterial infection influences the macrophage response and tissue regenerative processes.
Keywords: Mycobacterium marinum; infection; macrophages sub-types; necrosis; regeneration; zebrafish.
Copyright © 2022 Bohaud, Johansen, Varga, Contreras-Lopez, Barthelaix, Hamela, Sapède, Cloitre, Gergely, Jorgensen, Kremer and Djouad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Temporal modulation of host aerobic glycolysis determines the outcome of Mycobacterium marinum infection.Fish Shellfish Immunol. 2020 Jan;96:78-85. doi: 10.1016/j.fsi.2019.11.051. Epub 2019 Nov 24. Fish Shellfish Immunol. 2020. PMID: 31775059
-
RNAseq Profiling of Leukocyte Populations in Zebrafish Larvae Reveals a cxcl11 Chemokine Gene as a Marker of Macrophage Polarization During Mycobacterial Infection.Front Immunol. 2019 Apr 17;10:832. doi: 10.3389/fimmu.2019.00832. eCollection 2019. Front Immunol. 2019. PMID: 31110502 Free PMC article.
-
Priming of innate antimycobacterial immunity by heat-killed Listeria monocytogenes induces sterilizing response in the adult zebrafish tuberculosis model.Dis Model Mech. 2018 Jan 29;11(1):dmm031658. doi: 10.1242/dmm.031658. Dis Model Mech. 2018. PMID: 29208761 Free PMC article.
-
Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis.Cell Microbiol. 2008 May;10(5):1027-39. doi: 10.1111/j.1462-5822.2008.01133.x. Epub 2008 Feb 20. Cell Microbiol. 2008. PMID: 18298637 Review.
-
The Role of Macrophages During Zebrafish Injury and Tissue Regeneration Under Infectious and Non-Infectious Conditions.Front Immunol. 2021 Jul 21;12:707824. doi: 10.3389/fimmu.2021.707824. eCollection 2021. Front Immunol. 2021. PMID: 34367168 Free PMC article. Review.
Cited by
-
Inflammation in Development and Aging: Insights from the Zebrafish Model.Int J Mol Sci. 2024 Feb 10;25(4):2145. doi: 10.3390/ijms25042145. Int J Mol Sci. 2024. PMID: 38396822 Free PMC article. Review.
-
High species homology potentiates quantitative inflammation profiling in zebrafish using immunofluorescence.Heliyon. 2023 Dec 13;10(1):e23635. doi: 10.1016/j.heliyon.2023.e23635. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38187273 Free PMC article.
-
In vivo antimicrobial activity of engineered mesoporous silica nanoparticles targeting intracellular mycobacteria.Nat Commun. 2025 Aug 11;16(1):7388. doi: 10.1038/s41467-025-62623-y. Nat Commun. 2025. PMID: 40790109 Free PMC article.
-
Turning gray selenium and sublimed sulfur into a nanocomposite to accelerate tissue regeneration by isothermal recrystallization.J Nanobiotechnology. 2023 Feb 21;21(1):57. doi: 10.1186/s12951-023-01796-4. J Nanobiotechnology. 2023. PMID: 36803772 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

