Development and Validation of an Immune-Based Prognostic Risk Score for Patients With Resected Non-Small Cell Lung Cancer
- PMID: 35401554
- PMCID: PMC8983932
- DOI: 10.3389/fimmu.2022.835630
Development and Validation of an Immune-Based Prognostic Risk Score for Patients With Resected Non-Small Cell Lung Cancer
Abstract
Background: Despite the well-known role of immunoscore, as a prognostic tool, that appeared to be superior to tumor-node-metastasis (TNM) staging system, no prognostic scoring system based on immunohistochemistry (IHC) staining digital image analysis has been established in non-small cell lung cancer (NSCLC). Hence, we aimed to develop and validate an immune-based prognostic risk score (IMPRS) that could markedly improve individualized prediction of postsurgical survival in patients with resected NSCLC.
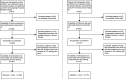
Methods: In this retrospective study, complete resection of NSCLC (stage I-IIIA) was performed for two independent patient cohorts (discovery cohort, n=168; validation cohort, n=115). Initially, paraffin-embedded resected specimens were stained by immunohistochemistry (IHC) of three immune cell types (CD3+, CD4+, and CD8+ T cells), and a total of 5,580 IHC-immune features were extracted from IHC digital images for each patient by using fully automated pipeline. Then, an IHC-immune signature was constructed with selected features using the LASSO Cox analysis, and the association of signature with patients' overall survival (OS) was analyzed by Kaplan-Meier method. Finally, IMPRS was established by incorporating IHC-immune signature and independent clinicopathological variables in multivariable Cox regression analysis. Furthermore, an external validation cohort was included to validate this prognostic risk score.
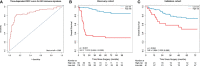
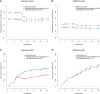
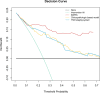
Results: Eight key IHC-immune features were selected for the construction of IHC-immune signature, which showed significant associations with OS in all cohorts [discovery: hazard ratio (HR)=11.518, 95%CI, 5.444-24.368; validation: HR=2.664, 95%CI, 1.029-6.896]. Multivariate analyses revealed IHC-immune signature as an independent prognostic factor, and age, T stage, and N stage were also identified and entered into IMPRS (all p<0.001). IMPRS had good discrimination ability for predicting OS (C-index, 0.869; 95%CI, 0.861-0.877), confirmed using external validation cohort (0.731, 0.717-0.745). Interestingly, IMPRS had better prognostic value than clinicopathological-based model and TNM staging system termed as C-index (clinicopathological-based model: 0.674; TNM staging: 0.646, all p<0.05). More importantly, decision curve analysis showed that IMPRS had adequate performance for predicting OS in resected NSCLC patients.
Conclusions: Our findings indicate that the IMPRS that we constructed can provide more accurate prognosis for individual prediction of OS for patients with resected NSCLC, which can help in guiding personalized therapy and improving outcomes for patients.
Keywords: immune-based prognostic risk score; immunohistochemistry; non-small cell lung cancer; overall survival; prognostic prediction.
Copyright © 2022 He, Huang, Chen, Huang, Wang, Zhang, Liang, Li, Yan and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Edge CC, BD SB, Fritz AG, Greene FL, Trotti A. eds. AJCC Cancer Staging Manual. 7th. New York: Springer; (2010).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

