Treating Patients With ANCA-Associated Vasculitis and Very Severe Renal Injury With an Intensified B Cell Depletion Therapy: Comparison With a Control Cohort Receiving a Conventional Therapy
- PMID: 35401565
- PMCID: PMC8988143
- DOI: 10.3389/fimmu.2022.777134
Treating Patients With ANCA-Associated Vasculitis and Very Severe Renal Injury With an Intensified B Cell Depletion Therapy: Comparison With a Control Cohort Receiving a Conventional Therapy
Abstract
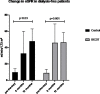
Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown to be an effective induction treatment for small-vessel vasculitides associated with antineutrophil cytoplasm antibodies (AAV) in both newly diagnosed and relapsing patients. However, the role of RTX in the management of the most severe cases of AAV remains to be fully elucidated. The aim of this study was to assess both safety and efficacy of an intensified B-cell depletion therapy (IBCDT) protocol, including RTX, cyclophosphamide (CYC), and methylprednisolone pulses without additional maintenance immunosuppressive therapy in a cohort of 15 AAV patients with the most severe features of AVV renal involvement (as <15 ml/min GFR and histological findings of paucimmune necrotizing glomerulonephritis with more than 50% crescents of non-sclerotic glomeruli at the renal biopsy). Results of the IBCDT regimen have been compared to those obtained in a control cohort of 10 patients with AAV treated with a conventional therapy regimen based on oral CYC and steroids followed by a prolonged maintenance therapy with azathioprine (AZA). Plasma exchange was equally employed in the study and the control group. Complete clinical remission (BVAS 0) was observed at 6 months in 14 of 15 patients treated with IBCDT (93%). All cases who achieved a complete clinical remission experienced a depletion of peripheral blood B cells at the end of therapy. Of the 10 dialysis dependent patients at onset, 6 subjects (60%) experienced a functional recovery allowing the suspension of dialysis treatment. When compared to the control group, no statistically significant difference was observed in patients treated with IBCDT in terms of overall survival, 6-month therapeutic response rate, and 6-, and 12-month functional renal recovery. The cumulative total dose of CYC in the case group was on average 1 g/patient while in the control group on average 8.5 g/patient (p = 0.00008). Despite the retrospective design and relative limited sample size, IBCDT appeared to be safe and had the same efficacy profile when compared to the conventional therapy with CYC plus AZA in the management of the most severe patients with AAV. Additionally, this avoided the need of prolonged maintenance therapy for long, and limited the exposure to CYC with consequent reduced toxicity and drug-related side effect rates.
Keywords: ANCA-associated vasculitis; glomerulonephritis; polyangiitis; polyangiitis and granulomatosis; rituximab; vasculitis.
Copyright © 2022 Roccatello, Sciascia, Murgia, Quattrocchio, Ferro, De Simone, Naretto, Barreca, Sammartino, Rossi and Fenoglio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and Validation of a Consensus Methodology for the Classification of the ANCA-Associated Vasculitides and Polyarteritis Nodosa for Epidemiological Studies. Ann Rheum Dis (2007) 66:222–27. doi: 10.1136/ard.2006.054593 - DOI - PMC - PubMed

