Enhanced Migratory Ability of Neutrophils Toward Epidermis Contributes to the Development of Psoriasis via Crosstalk With Keratinocytes by Releasing IL-17A
- PMID: 35401573
- PMCID: PMC8983831
- DOI: 10.3389/fimmu.2022.817040
Enhanced Migratory Ability of Neutrophils Toward Epidermis Contributes to the Development of Psoriasis via Crosstalk With Keratinocytes by Releasing IL-17A
Abstract
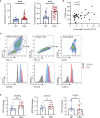
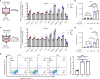
Microabscess of neutrophils in epidermis is one of the histological hallmarks of psoriasis. The axis of neutrophil-keratinocyte has been thought to play a critical role in the pathogenesis of psoriasis. However, the features and mechanism of interaction between the two cell types remain largely unknown. Herein, we found that blood neutrophils were increased in psoriasis patients, positively correlated with disease severity and highly expressed CD66b, but not CD11b and CD62L compared to healthy controls. Keratinocytes expressed high levels of psoriasis-related inflammatory mediators by direct and indirect interaction with neutrophils isolated from psoriasis patients and healthy controls. The capacity of neutrophils in provoking keratinocytes inflammatory response was comparable between the two groups and is dependent on IL-17A produced by itself. Neutrophils isolated from psoriasis patients displayed more transcriptome changes related to integrin and increased migration capacity toward keratinocytes with high CD11b expression on cell surface. Of interest, neutrophils were more susceptible to keratinocyte stimulation than to fibroblasts and human umbilical vein endothelial cells (HUVECs) in terms of CD11b expression and the production of ROS and NETs. In conclusion, neutrophils from psoriasis patients gain a strong capacity of IL-17A production and integrins expression that possibly facilitates their abilities to promote production of psoriasis-related inflammatory mediators and migration, a phenomenon likely induced by their interaction with keratinocytes but not with fibroblasts. These findings provide a proof-of-concept that development of new drugs targeting migration of neutrophils could be a more specific and safe solution to treat psoriasis.
Keywords: interleukin-17A; keratinocytes; migration; neutrophils; psoriasis.
Copyright © 2022 Liu, Shi, Lu, Hong, Qiu, Tan, Xiong, Guo and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Sumida H, Yanagida K, Kita Y, Abe J, Matsushima K, Nakamura M, et al. Interplay Between CXCR2 and BLT1 Facilitates Neutrophil Infiltration and Resultant Keratinocyte Activation in a Murine Model of Imiquimod-Induced Psoriasis. J Immunol (2014) 192(9):4361–9. doi: 10.4049/jimmunol.1302959 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

