Cardiovascular Dysfunction in COVID-19: Association Between Endothelial Cell Injury and Lactate
- PMID: 35401579
- PMCID: PMC8984030
- DOI: 10.3389/fimmu.2022.868679
Cardiovascular Dysfunction in COVID-19: Association Between Endothelial Cell Injury and Lactate
Abstract
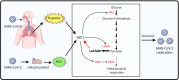
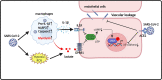
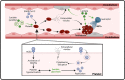
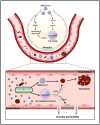
Coronavirus disease 2019 (COVID-19), an infectious respiratory disease propagated by a new virus known as Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), has resulted in global healthcare crises. Emerging evidence from patients with COVID-19 suggests that endothelial cell damage plays a central role in COVID-19 pathogenesis and could be a major contributor to the severity and mortality of COVID-19. Like other infectious diseases, the pathogenesis of COVID-19 is closely associated with metabolic processes. Lactate, a potential biomarker in COVID-19, has recently been shown to mediate endothelial barrier dysfunction. In this review, we provide an overview of cardiovascular injuries and metabolic alterations caused by SARS-CoV-2 infection. We also propose that lactate plays a potential role in COVID-19-driven endothelial cell injury.
Keywords: COVID-19; HMGB1 (High mobility group box 1); aerobic glycolytic metabolism; cardiovascular dysfunction; endothelial cell; lactate; thrombosis; vascular permeability.
Copyright © 2022 Yang, Holt, Fan, Lam, Yang, Ha, Williams, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Early spontaneous breathing for acute respiratory distress syndrome in individuals with COVID-19.Cochrane Database Syst Rev. 2022 Jun 29;6(6):CD015077. doi: 10.1002/14651858.CD015077. Cochrane Database Syst Rev. 2022. PMID: 35767435 Free PMC article.
Cited by
-
Risk factors affecting the development of pneumothorax in patients followed up in intensive care with a diagnosis of COVID-19.BMC Infect Dis. 2024 Nov 5;24(1):1243. doi: 10.1186/s12879-024-10147-z. BMC Infect Dis. 2024. PMID: 39501177 Free PMC article.
-
Metabolite profiles of diabetes mellitus and response to intervention in anti-hyperglycemic drugs.Front Endocrinol (Lausanne). 2023 Oct 31;14:1237934. doi: 10.3389/fendo.2023.1237934. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38027178 Free PMC article. Review.
-
Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction.Sci Adv. 2023 Feb 3;9(5):eadc9465. doi: 10.1126/sciadv.adc9465. Epub 2023 Feb 3. Sci Adv. 2023. PMID: 36735787 Free PMC article.
-
Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies.Acta Pharmacol Sin. 2023 Apr;44(4):695-709. doi: 10.1038/s41401-022-00998-0. Epub 2022 Oct 17. Acta Pharmacol Sin. 2023. PMID: 36253560 Free PMC article. Review.
-
Gen-miR-5 derived from Gentianella acuta inhibits PFKP to prevent fibroblast activation and alleviate myocardial fibrosis.Front Pharmacol. 2025 May 2;16:1578877. doi: 10.3389/fphar.2025.1578877. eCollection 2025. Front Pharmacol. 2025. PMID: 40385478 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

