Polyamine: A Potent Ameliorator for Plant Growth Response and Adaption to Abiotic Stresses Particularly the Ammonium Stress Antagonized by Urea
- PMID: 35401587
- PMCID: PMC8988247
- DOI: 10.3389/fpls.2022.783597
Polyamine: A Potent Ameliorator for Plant Growth Response and Adaption to Abiotic Stresses Particularly the Ammonium Stress Antagonized by Urea
Abstract
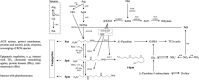
Polyamine(s) (PA, PAs), a sort of N-containing and polycationic compound synthesized in almost all organisms, has been recently paid considerable attention due to its multifarious actions in the potent modulation of plant growth, development, and response to abiotic/biotic stresses. PAs in cells/tissues occur mainly in free or (non- or) conjugated forms by binding to various molecules including DNA/RNA, proteins, and (membrane-)phospholipids, thus regulating diverse molecular and cellular processes as shown mostly in animals. Although many studies have reported that an increase in internal PA may be beneficial to plant growth under abiotic conditions, leading to a suggestion of improving plant stress adaption by the elevation of endogenous PA via supply or molecular engineering of its biosynthesis, such achievements focus mainly on PA homeostasis/metabolism rather than PA-mediated molecular/cellular signaling cascades. In this study, to advance our understanding of PA biological actions important for plant stress acclimation, we gathered some significant research data to succinctly describe and discuss, in general, PA synthesis/catabolism, as well as PA as an internal ameliorator to regulate stress adaptions. Particularly, for the recently uncovered phenomenon of urea-antagonized NH4 +-stress, from a molecular and physiological perspective, we rationally proposed the possibility of the existence of PA-facilitated signal transduction pathways in plant tolerance to NH4 +-stress. This may be a more interesting issue for in-depth understanding of PA-involved growth acclimation to miscellaneous stresses in future studies.
Keywords: G-protein-coupled receptor; abiotic stress; ammonium stress; lipid signaling; polyamine and arginine; urea signal.
Copyright © 2022 Sheng, Wu, Xiang, Pu, Duan, Huang, Cheng, Gong, Liang and Liu.
Conflict of interest statement
WP and PH were employed by company China Tobacco Hunan Industrial Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Antognoni F., Fornalè S., Grimmer C., Komor E., Bagni N. (1998). Long-distance translocation of polyamines in phloem and xylem of Ricinus communis L. plants. Planta 204 520–527. 10.1007/s004250050287 - DOI
Publication types
LinkOut - more resources
Full Text Sources

