The Plant Invertase/Pectin Methylesterase Inhibitor Superfamily
- PMID: 35401607
- PMCID: PMC8990755
- DOI: 10.3389/fpls.2022.863892
The Plant Invertase/Pectin Methylesterase Inhibitor Superfamily
Abstract
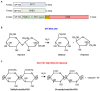
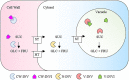
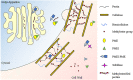
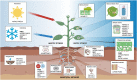
Invertases (INVs) and pectin methylesterases (PMEs) are essential enzymes coordinating carbohydrate metabolism, stress responses, and sugar signaling. INVs catalyzes the cleavage of sucrose into glucose and fructose, exerting a pivotal role in sucrose metabolism, cellulose biosynthesis, nitrogen uptake, reactive oxygen species scavenging as well as osmotic stress adaptation. PMEs exert a dynamic control of pectin methylesterification to manage cell adhesion, cell wall porosity, and elasticity, as well as perception and signaling of stresses. INV and PME activities can be regulated by specific proteinaceous inhibitors, named INV inhibitors (INVIs) and PME Inhibitors (PMEIs). Despite targeting different enzymes, INVIs and PMEIs belong to the same large protein family named "Plant Invertase/Pectin Methylesterase Inhibitor Superfamily." INVIs and PMEIs, while showing a low aa sequence identity, they share several structural properties. The two inhibitors showed mainly alpha-helices in their secondary structure and both form a non-covalent 1:1 complex with their enzymatic counterpart. Some PMEI members are organized in a gene cluster with specific PMEs. Although the most important physiological information was obtained in Arabidopsis thaliana, there are now several characterized INVI/PMEIs in different plant species. This review provides an integrated and updated overview of this fascinating superfamily, from the specific activity of characterized isoforms to their specific functions in plant physiology. We also highlight INVI/PMEIs as biotechnological tools to control different aspects of plant growth and defense. Some isoforms are discussed in view of their potential applications to improve industrial processes. A review of the nomenclature of some isoforms is carried out to eliminate confusion about the identity and the names of some INVI/PMEI member. Open questions, shortcoming, and opportunities for future research are also presented.
Keywords: CW integrity; biotechnological applications; degree of methylesterification; invertase inhibitors; pectin methylesterase inhibitors; plant growth and defence; sucrose metabolism.
Copyright © 2022 Coculo and Lionetti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- An S. H., Sohn K. H., Choi H. W., Hwang I. S., Lee S. C., Hwang B. K. (2008). Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 228, 61–78. doi: 10.1007/s00425-008-0719-z, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

