The Ovarian Transcriptome at the Early Stage of Testis Removal-Induced Male-To-Female Sex Change in the Protandrous Black Porgy Acanthopagrus schlegelii
- PMID: 35401660
- PMCID: PMC8986339
- DOI: 10.3389/fgene.2022.816955
The Ovarian Transcriptome at the Early Stage of Testis Removal-Induced Male-To-Female Sex Change in the Protandrous Black Porgy Acanthopagrus schlegelii
Abstract
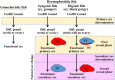
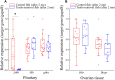
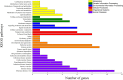
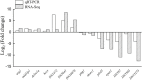
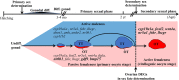
Unlike gonochoristic fishes, sex is fixed after gonadal differentiation (primary sex determination), and sex can be altered in adults (secondary sex determination) of hermaphroditic fish species. The secondary sex determination of hermaphroditic fish has focused on the differences between testicular tissue and ovarian tissue during the sex change process. However, comprehensive studies analyzing ovarian tissue or testicular tissue independently have not been performed. Hermaphroditic black porgy shows a digonic gonad (ovarian tissue with testicular tissue separated by connective tissue). Protandrous black porgy has stable maleness during the first two reproductive cycles (<2 years old), and approximately 50% enter femaleness (natural sex change) during the third reproductive cycle. Precocious femaleness is rarely observed in the estradiol-17β (E2)-induced female phase (oocytes maintained at the primary oocyte stage), and a reversible female-to-male sex change is found after E2 is withdrawn in <2-year-old fish. However, precocious femaleness (oocytes entering the vitellogenic oocyte stage) is observed in testis-removed fish in <2-year-old fish. We used this characteristic to study secondary sex determination (femaleness) in ovarian tissue via transcriptomic analysis. Cell proliferation analysis showed that BrdU (5-bromo-2'-deoxyuridine)-incorporated germline cells were significantly increased in the testis-removed fish (female) compared to the control (sham) fish (male) during the nonspawning season (2 months after surgery). qPCR analysis showed that there were no differences in pituitary-releasing hormones (lhb and gtha) in pituitary and ovarian steroidogenesis-related factors (star, cyp11a1, hsd3b1, and cyp19a1a) or female-related genes (wnt4a, bmp15, gdf9, figla, and foxl2) in ovarian tissues between intact and testis-removed fish (2 months after surgery). Low expression of pituitary fshb and ovarian cyp17a1 was found after 2 months of surgery. However, we did find small numbers of genes (289 genes) showing sexual fate dimorphic expression in both groups by transcriptomic analysis (1 month after surgery). The expression profiles of these differentially expressed genes were further examined by qPCR. Our present work identified several candidate genes in ovarian tissue that may be involved in the early period of secondary sex determination (femaleness) in black porgy. The data confirmed our previous suggestion that testicular tissue plays an important role in secondary sex determination in protandrous black porgy.
Keywords: estrogen; hermaphroditic fish; ovotestis; sex change; sex determination; sex differentiation; sexual fate.
Copyright © 2022 Tseng, Wu, Kuo, Tseng and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

