Genetic Parameter Estimation and Whole Sequencing Analysis of the Genetic Architecture of Chicken Keel Bending
- PMID: 35401685
- PMCID: PMC8984200
- DOI: 10.3389/fgene.2022.833132
Genetic Parameter Estimation and Whole Sequencing Analysis of the Genetic Architecture of Chicken Keel Bending
Abstract
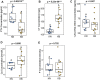
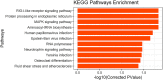
Bone health is particularly important for high-yielding commercial layer chickens. The keel of poultry is an extension of the abdomen side of the sternum along the sagittal plane and is one of the most important bones. In this study, the keel phenotype of White Leghorns laying hen flocks showed significant individual differences. To clarify its genetic mechanism, we first estimated the heritability of keel bend (KB) in White Leghorn, recorded the production performance of the chicken flock, examined the blood biochemical indexes and bone quality in KB and keel normal (KN) chickens, and performed whole-genome pooled sequencing in KB and KN chickens. We then performed selection elimination analysis to determine the genomic regions that may affect the keel phenotypes. The results show that KB is a medium heritability trait. We found that cage height had a significant effect on the KB (p < 0.01). At 48 weeks, there were significant differences in the number of eggs, the number of normal eggs, and eggshell strength (p < 0.05). The content of parathyroid hormone was lower (p < 0.01) and that of calcitonin was higher (p < 0.01) in KB chickens than in KN chickens. The differences in bone mineral density, bone strength, and bone cortical thickness of the humerus and femur were extremely significant (p < 0.01), with all being lower in KB chickens than in KN chickens. In addition, the bones of KB chickens contained more fat organization. A total of 128 genes were identified in selective sweep regions. We identified 10 important candidate genes: ACP5, WNT1, NFIX, CNN1, CALR, FKBP11, TRAPPC5, MAP2K7, RELA, and ENSGALG00000047166. Among the significantly enriched Kyoto Encyclopedia of Genes and Genomes pathways found, we identifed two bone-related pathways, one involving "osteoclast differentiation" and the other the "MAPK signaling pathway." These results may help us better understand the molecular mechanism of bone traits in chickens and other birds and provide new insights for the genetic breeding of chickens.
Keywords: candidate gene; chicken; genetic parameters; keel bend; pool-seq.
Copyright © 2022 Zhang, Yang, Zhu, Wang, Zhao, Zhao, Qu and Jia.
Conflict of interest statement
Authors XZ and GZ were employed by the company Hebei Dawu Poultry Breeding Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

