MUC21 induces the viability and migration of glioblastoma via the STAT3/AKT pathway
- PMID: 35401801
- PMCID: PMC8987941
- DOI: 10.3892/etm.2022.11260
MUC21 induces the viability and migration of glioblastoma via the STAT3/AKT pathway
Abstract
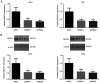
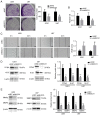
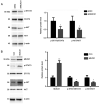
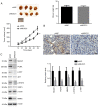
Glioblastoma (GBM) is a malignant tumor with one of the fastest increasing morbidity and mortality rates. As such, more therapeutic targets need to be developed to combat this disease. Mucin 21 (MUC21) is a human counterpart of mouse epiglycanin and mediates multiple cellular functions. However, its possible effects on GBM and its possible mechanism remain unclear. The current study aimed to clarify the role or MUC21 in the progression of GBM by performing a series of in vitro assays, including Cell Counting Kit-8, colony formation, wound closure, transwell, and in vivo assays. In the present study, the aberrantly high expression of MUC21 in human GBM tissues and cell lines was observed and it was revealed that it was associated with the clinicopathological feature, tumor recurrence, in patients with GBM. MUC21 promoted the viability and motility of GBM cells in vitro and stimulated tumor growth in vivo. It was further confirmed that MUC21 promoted the progression of GBM via the STAT3/AKT pathway and it was considered that MUC21 could serve as a promising target for the treatment of GBM.
Keywords: STAT3/AKT pathway; glioblastoma; mucin 21; prognosis; vaibility.
Copyright: © Wang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
MUC21: a new target for tumor treatment.Front Oncol. 2024 Jun 12;14:1410761. doi: 10.3389/fonc.2024.1410761. eCollection 2024. Front Oncol. 2024. PMID: 38933439 Free PMC article. Review.
-
Overexpression of HOXC10 promotes glioblastoma cell progression to a poor prognosis via the PI3K/AKT signalling pathway.J Drug Target. 2019 Jan;27(1):60-66. doi: 10.1080/1061186X.2018.1473408. Epub 2018 Aug 6. J Drug Target. 2019. PMID: 29768063
-
MUC21 controls melanoma progression via regulating SLITRK5 and hedgehog signaling pathway.Cell Biol Int. 2022 Sep;46(9):1458-1467. doi: 10.1002/cbin.11817. Epub 2022 May 17. Cell Biol Int. 2022. PMID: 35579188
-
Polydatin executes anticancer effects against glioblastoma multiforme by inhibiting the EGFR-AKT/ERK1/2/STAT3-SOX2/Snail signaling pathway.Life Sci. 2020 Oct 1;258:118158. doi: 10.1016/j.lfs.2020.118158. Epub 2020 Aug 1. Life Sci. 2020. PMID: 32750435
-
Roles of STAT3 in the pathogenesis and treatment of glioblastoma.Front Cell Dev Biol. 2023 Feb 27;11:1098482. doi: 10.3389/fcell.2023.1098482. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36923251 Free PMC article. Review.
Cited by
-
MUC21: a new target for tumor treatment.Front Oncol. 2024 Jun 12;14:1410761. doi: 10.3389/fonc.2024.1410761. eCollection 2024. Front Oncol. 2024. PMID: 38933439 Free PMC article. Review.
-
GDNF-induced phosphorylation of MUC21 promotes pancreatic cancer perineural invasion and metastasis by activating RAC2 GTPase.Oncogene. 2024 Aug;43(34):2564-2577. doi: 10.1038/s41388-024-03102-4. Epub 2024 Jul 17. Oncogene. 2024. PMID: 39020072
-
A CRISPR activation screen identifies MUC-21 as critical for resistance to NK and T cell-mediated cytotoxicity.J Exp Clin Cancer Res. 2023 Oct 20;42(1):272. doi: 10.1186/s13046-023-02840-9. J Exp Clin Cancer Res. 2023. PMID: 37858248 Free PMC article.
-
Thioflavin T Lasing Probe for Mucin Detection in Simulated Tears as a Targeting Strategy for Brain Tumors.ACS Chem Neurosci. 2025 Jul 2;16(13):2371-2375. doi: 10.1021/acschemneuro.5c00274. Epub 2025 May 15. ACS Chem Neurosci. 2025. PMID: 40372412 Free PMC article.
-
Mucins as Precision Biomarkers in Glioma: Emerging Evidence for Their Potential in Biospecimen Analysis and Outcome Prediction.Biomedicines. 2024 Dec 11;12(12):2806. doi: 10.3390/biomedicines12122806. Biomedicines. 2024. PMID: 39767713 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
