Wound microenvironment-responsive glucose consumption and hydrogen peroxide generation synergistic with azithromycin for diabetic wounds healing
- PMID: 35401823
- PMCID: PMC8965477
- DOI: 10.7150/thno.64244
Wound microenvironment-responsive glucose consumption and hydrogen peroxide generation synergistic with azithromycin for diabetic wounds healing
Abstract
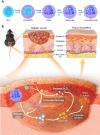
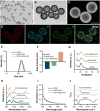
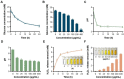
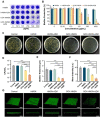
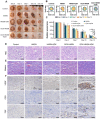
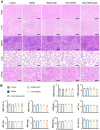
Rationale: Chronic wounds are one of the common complications of diabetes. Due to the physiological conditions of diabetic patients, these wounds are more susceptible to bacterial infections and the formation of bacterial biofilms, leading to the inefficiency of conventional antibiotic treatment. Methods: Here, hollow mesoporous silica nanoparticles (HMSN) were used as the nanocarriers for co-delivery of azithromycin (AZM) and glucose oxidase (GOX), achieving a remarkable synergistic effect in chronic diabetic wounds. GOX possesses the catalytic ability to consume glucose and produce H2O2 in the diabetic wound area. The down-regulation of local glucose could effectively improve the chronic diabetic wound microenvironment. Meanwhile, the generated H2O2 effectively inhibits bacterial growth and eradicates bacterial biofilms with the synergism of antibiotics AZM. Results: In the bacteria-infected diabetic cutaneous wound models, the reduction of glucose, generation of H2O2, and release of AZM could effectively reduce the bacterial infection and promote the wounds healing. Moreover, there is no obvious toxicity behavior after the treatment. Conclusions: Therefore, the designed nanosystem could effectively accelerate the diabetic wound healing process by the amelioration of the hyperglycemia microenvironment and the eradication of bacterial biofilms around the wounds, making them promising candidates for clinical transformation.
Keywords: azithromycin; bacterial biofilm; diabetic wound healing; glucose oxidase; silica nanoparticle.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Cho H, Blatchley MR, Duh EJ, Gerecht S. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev. 2019;146:267–288. - PubMed
-
- Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. - PubMed
-
- Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–2923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

