Cold atmospheric plasma for preventing infection of viruses that use ACE2 for entry
- PMID: 35401827
- PMCID: PMC8965494
- DOI: 10.7150/thno.70098
Cold atmospheric plasma for preventing infection of viruses that use ACE2 for entry
Abstract
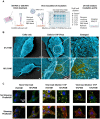
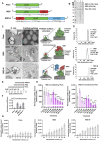
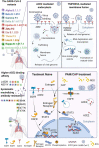
Rational: The mutating SARS-CoV-2 potentially impairs the efficacy of current vaccines or antibody-based treatments. Broad-spectrum and rapid anti-virus methods feasible for regular epidemic prevention against COVID-19 or alike are urgently called for. Methods: Using SARS-CoV-2 virus and bioengineered pseudoviruses carrying ACE2-binding spike protein domains, we examined the efficacy of cold atmospheric plasma (CAP) on virus entry prevention. Results: We found that CAP could effectively inhibit the entry of virus into cells. Direct CAP or CAP-activated medium (PAM) triggered rapid internalization and nuclear translocation of the virus receptor, ACE2, which began to return after 5 hours and was fully recovered by 12 hours. This was seen in vitro with both VERO-E6 cells and human mammary epithelial MCF10A cells, and in vivo. Hydroxyl radical (·OH) and species derived from its interactions with other species were found to be the most effective CAP components for triggering ACE2 nucleus translocation. The ERα/STAT3(Tyr705) and EGFR(Tyr1068/1086)/STAT3(Tyr705) axes were found to interact and collectively mediate the effects on ACE2 localization and expression. Conclusions: Our data support the use of PAM in helping control SARS-CoV-2 if developed into products for nose/mouth spray; an approach extendable to other viruses utilizing ACE2 for host entry.
Keywords: Angiotensin-converting enzyme 2; Plasma activated medium (PAM); SARS-CoV-2; cold atmospheric plasma (CAP).
© The author(s).
Conflict of interest statement
Competing Interests: BHAR is co-founder and shareholder of PolyBatics Ltd. The other authors declare no competing financial interest.
Figures
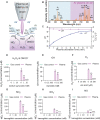
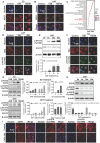
 , and
, and  . The Y axis shows fluorescence intensity (arbitrary units).
. The Y axis shows fluorescence intensity (arbitrary units).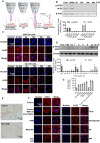
Similar articles
-
Use of recombinant S1 protein with hFc for analysis of SARS-CoV-2 adsorption and evaluation of drugs that inhibit entry into VERO E6 cells.Immunol Lett. 2023 Nov;263:105-112. doi: 10.1016/j.imlet.2023.09.002. Epub 2023 Sep 6. Immunol Lett. 2023. PMID: 37683695
-
Multidisciplinary Approaches Identify Compounds that Bind to Human ACE2 or SARS-CoV-2 Spike Protein as Candidates to Block SARS-CoV-2-ACE2 Receptor Interactions.mBio. 2021 Mar 30;12(2):e03681-20. doi: 10.1128/mBio.03681-20. mBio. 2021. PMID: 33785634 Free PMC article.
-
Potential therapeutic approaches for the early entry of SARS-CoV-2 by interrupting the interaction between the spike protein on SARS-CoV-2 and angiotensin-converting enzyme 2 (ACE2).Biochem Pharmacol. 2021 Oct;192:114724. doi: 10.1016/j.bcp.2021.114724. Epub 2021 Aug 8. Biochem Pharmacol. 2021. PMID: 34371003 Free PMC article. Review.
-
ACE2-Decorated Virus-Like Particles Effectively Block SARS-CoV-2 Infection.Int J Nanomedicine. 2024 Jul 10;19:6931-6943. doi: 10.2147/IJN.S446093. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39005960 Free PMC article.
-
The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target.Tumour Biol. 2021;43(1):177-196. doi: 10.3233/TUB-200084. Tumour Biol. 2021. PMID: 34420993 Review.
Cited by
-
Immunological Control of Herpes Simplex Virus Type 1 Infection: A Non-Thermal Plasma-Based Approach.Viruses. 2025 Apr 23;17(5):600. doi: 10.3390/v17050600. Viruses. 2025. PMID: 40431612 Free PMC article. Review.
-
Oral SARS-CoV-2 reduction by local treatment: A plasma technology application?Plasma Process Polym. 2022 Dec 22:e2200196. doi: 10.1002/ppap.202200196. Online ahead of print. Plasma Process Polym. 2022. PMID: 36721423 Free PMC article.
-
Toxicity and virucidal activity of a neon-driven micro plasma jet on eukaryotic cells and a coronavirus.Free Radic Biol Med. 2022 Oct;191:105-118. doi: 10.1016/j.freeradbiomed.2022.08.026. Epub 2022 Aug 28. Free Radic Biol Med. 2022. PMID: 36041652 Free PMC article.
-
Nebulized inhalation of plasma-activated water in the treatment of progressive moderate COVID-19 patients with antiviral treatment failure: a randomized controlled pilot trial.BMC Infect Dis. 2024 Sep 12;24(1):960. doi: 10.1186/s12879-024-09886-w. BMC Infect Dis. 2024. PMID: 39266946 Free PMC article. Clinical Trial.
-
Interaction Mechanisms of Cold Atmospheric Plasmas with HIV Capsid Protein by Reactive Molecular Dynamics Simulation.Molecules. 2024 Dec 30;30(1):101. doi: 10.3390/molecules30010101. Molecules. 2024. PMID: 39795158 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

