AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages
- PMID: 35401830
- PMCID: PMC8965475
- DOI: 10.7150/thno.69533
AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages
Abstract
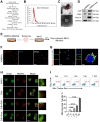
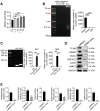
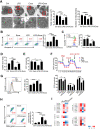
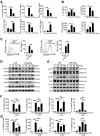
Rationale: Aberrant activation of macrophages with mitochondria dismiss was proved to be associated with pathogenesis of ALI (acute lung injury). Exosomes from adipose-derived mesenchymal stem cells (AdMSC-Exos) have been distinguished by their low immunogenicity, lack of tumorigenicity, and high clinical safety, but their role in treating ALI and the mechanism involved need to be defined. In this study, we sought to investigate whether the mitochondrial donation from AdMSC-Exos provides profound protection against LPS-induced ALI in mice, accompanied by improvement of macrophage mitochondrial function. Methods: C57BL/6 mice were orotracheally instilled with LPS (1 mg/kg). AdMSC-Exos were administered via the tail vein 4 h after LPS inhalation. Flow cytometry, H&E, Quantitative Real-Time PCR, immunofluorescence (IF), confocal microscopy imaging was conducted to investigate lung tissue inflammation and macrophage mitochondrial function. And further observe the transfer of exosomes and the effect on mitochondrial function of MH-S cells through in vitro experiments. Results: AdMSC-Exos can transfer the stem cell-derived mitochondria components to alveolar macrophages in a dose-dependent manner. Likely through complementing the damaged mitochondria, AdMSC-Exos exhibited the ability to elevate the level of mtDNA, mitochondrial membrane potential (MMP), OXPHOS activity and ATP generation, while reliving mROS stress in LPS-challenged macrophages. Restoring mitochondrial integrity via AdMSC-Exos treatment enabled macrophages shifting to anti-inflammatory phenotype, as featured with the down-regulation of IL-1β, TNF-α and iNOS secretion and increase in production of anti-inflammatory cytokines IL-10 and Arg-1. As we depleted alveolar macrophages using clodronate liposomes, the protective role for AdMSC-Exos was largely abrogated. Conclusions: AdMSC-Exos can effectively donate mitochondria component improved macrophages mitochondrial integrity and oxidative phosphorylation level, leading to the resumption of metabolic and immune homeostasis of airway macrophages and mitigating lung inflammatory pathology.
Keywords: acute lung injury; alveolar macrophages; exosomes; mitochondrial function; stem cells.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Sapoznikov A, Gal Y, Falach R, Sagi I, Ehrlich S, Lerer E. et al. Early disruption of the alveolar-capillary barrier in a ricin-induced ARDS mouse model: neutrophil-dependent and-independent impairment of junction proteins. Am J Physiol Lung Cell Mol Physiol. 2019;316:255–68. - PubMed
-
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A. et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. - PubMed
-
- Pham T, Rubenfeld GD. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome: a 50th birthday review. Am J Respir Crit Care Med. 2017;195:860–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

