Recent progress of carbon dots in targeted bioimaging and cancer therapy
- PMID: 35401835
- PMCID: PMC8965501
- DOI: 10.7150/thno.70721
Recent progress of carbon dots in targeted bioimaging and cancer therapy
Abstract
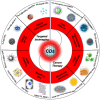
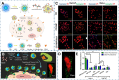
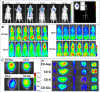
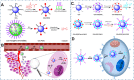
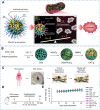
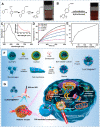
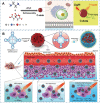
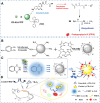
Carbon dots (CDs), as one new class of carbon nanomaterials with various structure and extraordinary physicochemical properties, have attracted tremendous interest for their potential applications in tumor theranostics, especially in targeted bioimaging and therapy. In these areas, CDs and its derivatives have been employed as highly efficient imaging agent for photoluminescence bioimaging of tumors cells. With unique structure, optical and/or dose attention properties, CDs have been harnessed in various nanotheranostic strategies for diverse tumors through integrating with other functional nanoparticles or utilizing their inherent physical properties. Up to now, CDs have been approved as novel biomaterials by their excellent performances in precise targeted bioimaging and therapy for tumors. Herein, the latest progress in the development of CDs in targeted bioimaging and tumor therapy are reviewed. Meanwhile, the challenges and future prospects of the application of CDs in promising nanotheranostic strategies are discussed and proposed.
Keywords: Carbon dots; cancer therapy; nanotheranostic; targeted bioimaging.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures










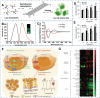
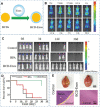

Similar articles
-
Carbon Dots for In Vivo Bioimaging and Theranostics.Small. 2019 Aug;15(32):e1805087. doi: 10.1002/smll.201805087. Epub 2019 Feb 18. Small. 2019. PMID: 30779301 Review.
-
Progress on Carbon Dots with Intrinsic Bioactivities for Multimodal Theranostics.Adv Healthc Mater. 2025 Jan;14(1):e2402285. doi: 10.1002/adhm.202402285. Epub 2024 Oct 23. Adv Healthc Mater. 2025. PMID: 39440645 Review.
-
New insight into the engineering of green carbon dots: Possible applications in emerging cancer theranostics.Talanta. 2020 Mar 1;209:120547. doi: 10.1016/j.talanta.2019.120547. Epub 2019 Nov 13. Talanta. 2020. PMID: 31892009 Review.
-
2D Nanomaterials for Cancer Theranostic Applications.Adv Mater. 2020 Apr;32(13):e1902333. doi: 10.1002/adma.201902333. Epub 2019 Jul 28. Adv Mater. 2020. PMID: 31353752 Review.
-
Regulating photochemical properties of carbon dots for theranostic applications.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023 May-Jun;15(3):e1862. doi: 10.1002/wnan.1862. Epub 2022 Nov 8. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023. PMID: 36347269 Review.
Cited by
-
Innovative applications of advanced nanomaterials in cerebrovascular imaging.Front Bioeng Biotechnol. 2025 Jan 22;12:1456704. doi: 10.3389/fbioe.2024.1456704. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39911816 Free PMC article. Review.
-
N-doped carbon dots for dual-modality NIR fluorescence imaging and photothermal therapy.J Nanobiotechnology. 2025 Jul 15;23(1):513. doi: 10.1186/s12951-025-03497-6. J Nanobiotechnology. 2025. PMID: 40660215 Free PMC article.
-
The Role of Ion Channels in Cervical Cancer Progression: From Molecular Biomarkers to Diagnostic and Therapeutic Innovations.Cancers (Basel). 2025 May 1;17(9):1538. doi: 10.3390/cancers17091538. Cancers (Basel). 2025. PMID: 40361464 Free PMC article. Review.
-
Theranostic Applications of Taurine-Derived Carbon Dots in Colorectal Cancer: Ferroptosis Induction and Multifaceted Antitumor Mechanisms.Int J Nanomedicine. 2025 Jun 16;20:7613-7635. doi: 10.2147/IJN.S516926. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40546797 Free PMC article.
-
Review on Carbon Dot-Based Fluorescent Detection of Biothiols.Biosensors (Basel). 2023 Mar 2;13(3):335. doi: 10.3390/bios13030335. Biosensors (Basel). 2023. PMID: 36979547 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394–424. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15:385–403. - PubMed
-
- De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

