Multimodal cortico-cortical associations induced by fear and sensory conditioning in the guinea pig
- PMID: 35401874
- PMCID: PMC8934902
- DOI: 10.1007/s11571-021-09708-8
Multimodal cortico-cortical associations induced by fear and sensory conditioning in the guinea pig
Abstract
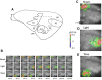
Sensory cortices are defined by responses to physical stimulation in specific modalities. Recently, additional associatively induced responses have been reported for stimuli other than the main specific modality for each cortex in the human and mammalian brain. In this study, to investigate a type of consolidation, associative responses in the guinea pig cortices (auditory, visual, and somatosensory) were simultaneously measured using optical imaging after first- or second-order conditioning comprising foot shock as an aversive stimulus and tone and light as sensory stimuli. Our findings indicated that (1) after the first- and second-order conditioning, associative responses in each cortical area were additionally induced to stimulate the other specific modality; (2) an associative response to sensory conditioning with tone and light was also seen as a change in the response at the neuronal level without behavioral phenomena; and (3) when fear conditioning with light and foot shock was applied before sensory conditioning with tone and light, the associative response to foot shock in the primary visual cortex (V1) was decreased (extinction) compared with the response after the first-order fear conditioning, whereas the associative response was increased (facilitation) for fear conditioning after sensory conditioning. Our results suggest that various types of bottom-up information are consolidated as associative responses induced in the cortices, which are traced repetitively or alternatively by a change in plasticity involving facilitation and extinction in the cortical network. This information-combining process of cortical responses may play a crucial role in the dynamic linking of memory in the brain.
Keywords: Associative response; Guinea pig; Higher-order conditioning; Optical imaging; Sensory cortex.
© The Author(s), under exclusive licence to Springer Nature B.V. 2021.
Figures




References
LinkOut - more resources
Full Text Sources

