The Benefits and Risks of Iron interventionS in Children (BRISC) trial: Statistical analysis plan
- PMID: 35401970
- PMCID: PMC8984212
- DOI: 10.12688/f1000research.23383.1
The Benefits and Risks of Iron interventionS in Children (BRISC) trial: Statistical analysis plan
Abstract
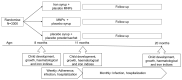
Background: The Benefits and Risks of Iron interventionS in Children (BRISC) trial will evaluate the impact of universal supplementation with iron supplements or iron-containing multiple micronutrient powders (MNPs) compared with placebo given for 3 months on child development, growth, morbidity, laboratory indices of anaemia, iron deficiency, and inflammation at end of intervention and after a further 9 months post intervention in children aged 8 months living in rural Bangladesh. This paper describes the statistical analysis plan. Methods: BRISC is a multi-site, three-arm, double-dummy blinded, parallel group, randomised control superiority trial in 3300 children. The statistical analysis plan was developed by the trial statistician in consultation with the trial steering committee and trial management committee based on the protocol, data collection forms, and study outcomes available in the blinded study database. Conclusion: This detailed statistical analysis plan published prior to unblinding the allocated treatments will support the statistical analyses and reporting of the BRISC trial to be undertaken after unblinding. It allows for transparency as well as reproducibility of statistical analyses and reporting. Registration: Australian New Zealand Clinical Trials Registry ACTRN12617000660381 (registered on 8 May 2017); World Health Organization Universal Trial Number U1111-1196-1125.
Keywords: Bangladesh; Bayley Scales; Iron; anaemia; child development; cognition; randomised control trial; statistical analysis plan.
Copyright: © 2020 Braat S et al.
Conflict of interest statement
No competing interests were disclosed.
Figures
References
-
- World Health Organization: Guideline: daily iron supplementation in infants and children.Geneva: World Health Organization.2016. Accessed Jan 2018. Reference Source - PubMed
-
- World Health Organization: WHO guideline: Use of multiple micronutrient powders for point-of-use fortication of foods consumed by infants and young children aged 6– 23 months and children aged 2– 12 years.Geneva: World Health Organization,2016. Reference Source - PubMed
LinkOut - more resources
Full Text Sources