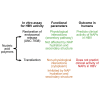
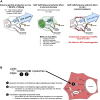
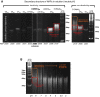
Editorial: In vitro mechanistic evaluation of nucleic acid polymers: A cautionary tale
- PMID: 35402067
- PMCID: PMC8956958
- DOI: 10.1016/j.omtn.2022.03.002
Editorial: In vitro mechanistic evaluation of nucleic acid polymers: A cautionary tale
Keywords: hepatitis B; mechanism of action; nucleic acid polymer; oligonucleotide; transfection artifacts.
Conflict of interest statement
A.V. is an employee and shareholder of Replicor Inc.
Figures



References
-
- Vaillant A. Nucleic acid polymers: broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antivir. Res. 2016;133:32–40. - PubMed
-
- Vaillant A. Rep 2139: antiviral mechanisms and applications in achieving functional control of HBV and HDV infection. ACS Infect Dis. 2019;5:675–687. - PubMed
-
- Bazinet M., Pantea V., Placinta G., Moscalu I., Cebotarescu V., Cojuhari L., Jimbei P., Iarovoi L., Smesnoi V., Musteata T., et al. Safety and efficacy of 48 Weeks REP 2139 or REP 2165, tenofovir disoproxil, and pegylated interferon alfa-2a in patients with chronic HBV infection naive to nucleos(t)ide therapy. Gastroenterology. 2020;158:2180–2194. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

