LncRNA B4GALT1-AS1 promotes non-small cell lung cancer cell growth via increasing ZEB1 level by sponging miR-144-3p
- PMID: 35402178
- PMCID: PMC8990218
- DOI: 10.21037/tcr-22-296
LncRNA B4GALT1-AS1 promotes non-small cell lung cancer cell growth via increasing ZEB1 level by sponging miR-144-3p
Abstract
Background: Long noncoding RNAs (lncRNAs) are emerging as key players in the development and progression of cancer. Several malignancies involve dysregulated long noncoding ribonucleic acids (lncRNAs) in non-small cell lung cancer cell growth and their aggressive phenotypes. LncRNA B4GALT1-AS1 is important in the advancement of various malignancies, although its contribution to non-small cell lung cancer (NSCLC) remains unexplored.
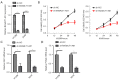
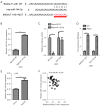
Methods: LncRNA B4GALT1-AS1 in NSCLC tissues was detected and further validated in a cohort of non-small cell lung cancer tissues. The effects of lncRNA B4GALT1-AS1 on proliferation were determined by in vitro experiments. The B4GALT1-AS1-miR-144-3p-ZEB1 axis was assessed by dual-luciferase reporter and RNA immunoprecipitation (RIP) assays. Furthermore, the mechanism of B4GALT1-AS1 was investigated using loss-of-function assays in vitro.
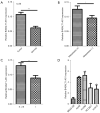
Results: We showed significant upregulation of B4GALT1-AS1 in cell lines and tissues of NSCLC. B4GALT1-AS1 knockdown impeded the in vitro proliferation-related characteristics of the NSCLC cells. The demonstration of the binding capacity of B4GALT1-AS1 and miR-144-3p was predicted by bioinformatics and luciferase reporter activity assay. The B4GALT1-AS1 and miR-144-3p interaction was shown by using rescue experiments. NSCLC has a positive association with its target, zinc finger e-box binding homeobox 1 (ZEB1).
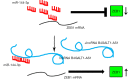
Conclusions: In summary, the progression of NSCLC was facilitated by lncRNA B4GALT1-AS1 via interaction with miR-144-3p and positive regulation of ZEB1 expression.
Keywords: B4GALT1-AS1; Zinc finger e-box binding homeobox 1 (ZEB1); metastasis; miR-144-3p; non-small cell lung cancer (NSCLC).
2022 Translational Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-296/coif). The authors have no conflicts of interest to declare.
Figures

Similar articles
-
LncRNA ZEB1-AS1/miR-409-3p/ZEB1 feedback loop is involved in the progression of non-small cell lung cancer.Biochem Biophys Res Commun. 2018 Dec 9;507(1-4):450-456. doi: 10.1016/j.bbrc.2018.11.059. Epub 2018 Nov 15. Biochem Biophys Res Commun. 2018. PMID: 30448056
-
Thalidomide suppresses angiogenesis and immune evasion via lncRNA FGD5-AS1/miR-454-3p/ZEB1 axis-mediated VEGFA expression and PD-1/PD-L1 checkpoint in NSCLC.Chem Biol Interact. 2021 Nov 1;349:109652. doi: 10.1016/j.cbi.2021.109652. Epub 2021 Sep 11. Chem Biol Interact. 2021. PMID: 34520751
-
LncRNA NNT-AS1 promotes non-small cell lung cancer progression through regulating miR-22-3p/YAP1 axis.Thorac Cancer. 2020 Mar;11(3):549-560. doi: 10.1111/1759-7714.13280. Epub 2020 Jan 10. Thorac Cancer. 2020. PMID: 31923353 Free PMC article.
-
Long non-coding RNA B4GALT1-Antisense RNA 1/microRNA-30e/SRY-box transcription factor 9 signaling axis contributes to non-small cell lung cancer cell growth.Oncol Lett. 2020 Dec;20(6):284. doi: 10.3892/ol.2020.12146. Epub 2020 Sep 23. Oncol Lett. 2020. PMID: 33014162 Free PMC article.
-
A review on the role of ZEB1-AS1 in human disorders.Pathol Res Pract. 2023 May;245:154486. doi: 10.1016/j.prp.2023.154486. Epub 2023 Apr 26. Pathol Res Pract. 2023. PMID: 37120907 Review.
Cited by
-
LncRNA HOXD-AS2 regulates miR-3681-5p/DCP1A axis to promote the progression of non-small cell lung cancer.J Thorac Dis. 2023 Mar 31;15(3):1289-1301. doi: 10.21037/jtd-23-153. Epub 2023 Mar 27. J Thorac Dis. 2023. PMID: 37065560 Free PMC article.
-
Circular RNAs in EMT-driven metastasis regulation: modulation of cancer cell plasticity, tumorigenesis and therapy resistance.Cell Mol Life Sci. 2024 May 11;81(1):214. doi: 10.1007/s00018-024-05236-w. Cell Mol Life Sci. 2024. PMID: 38733529 Free PMC article. Review.
-
miR-144-3p Targets GABRB2 to Suppress Thyroid Cancer Progression In Vitro.Cell Biochem Biophys. 2024 Dec;82(4):3585-3595. doi: 10.1007/s12013-024-01446-y. Epub 2024 Aug 2. Cell Biochem Biophys. 2024. PMID: 39093515
-
Exosome-mediated circGMPS facilitates the development of gastric cancer cells through miR-144-3p/PUM1.Cytotechnology. 2024 Feb;76(1):53-68. doi: 10.1007/s10616-023-00597-9. Epub 2023 Oct 20. Cytotechnology. 2024. PMID: 38304630 Free PMC article.
-
Downregulation of long noncoding RNA B4GALT1-AS1 is associated with breast cancer development.Sci Rep. 2024 Feb 7;14(1):3114. doi: 10.1038/s41598-023-51124-x. Sci Rep. 2024. PMID: 38326326 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
