Susceptibility-Guided Therapy vs. Bismuth-Containing Quadruple Therapy as the First-Line Treatment for Helicobacter pylori Infection: A Systematic Review and Meta-Analysis
- PMID: 35402425
- PMCID: PMC8987208
- DOI: 10.3389/fmed.2022.844915
Susceptibility-Guided Therapy vs. Bismuth-Containing Quadruple Therapy as the First-Line Treatment for Helicobacter pylori Infection: A Systematic Review and Meta-Analysis
Abstract
Background: The increased antibiotic resistance of Helicobacter pylori (H. pylori) has led to the decreased efficacy of H. pylori regimens.
Aim: To evaluate the efficacy, safety, and compliance of susceptibility-guided therapy (SGT) vs. bismuth-containing quadruple therapy (BQT) as the first-line treatment for H. pylori infection.
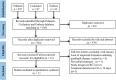
Materials and methods: This meta-analysis was performed in accordance with the PRISMA 2009 guidelines. A systematic search in PubMed, Embase, and Cochrane databases was conducted using the combination of "H. pylori or H. pylori or Hp," "bismuth quadruple," and "tailored eradication OR tailored therapy OR susceptibility-guided therapy OR personalized therapy OR antibiotic susceptibility testing."
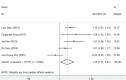
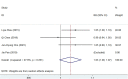
Results: Five studies with 2,110 H. pylori-infected patients were enrolled. The pooled eradication rates of SGT and BQT were 86 vs. 78% (p < 0.05) and 92 vs. 86% (p > 0.05) by intention-to-treat (ITT) and per-protocol (PP) analyses, respectively. SGT has a significantly superior efficacy than BQT [pooled risk ratio (RR) = 1.14, p < 0.05] in a subgroup of cultures with the susceptibility test. The pooled side effect rate was 20% in SGT and 22% in BQT, which showed no significant difference (p > 0.05). The compliances of SGT and BQT were 95 and 92%, respectively.
Conclusion: Compared with BQT, SGT showed a higher efficacy and similar safety as the first-line treatment of H. pylori infection in areas with high antibiotic resistance. The decision-making of first-line regimens for H. pylori infection should depend on the availability and cost-effectiveness of susceptibility tests and bismuth in local areas.
Keywords: Helicobacter pylori; bismuth containing quadruple therapy; efficacy; meta-analysis; susceptibility-guided therapy.
Copyright © 2022 Ouyang, Zhang, He, Zhu, Lu and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Bismuth-containing quadruple therapy versus concomitant quadruple therapy as first-line treatment for Helicobacter Pylori infection in an area of high resistance to clarithromycin: A prospective, cross-sectional, comparative, open trial.Helicobacter. 2019 Feb;24(1):e12546. doi: 10.1111/hel.12546. Epub 2018 Oct 22. Helicobacter. 2019. PMID: 30346636 Clinical Trial.
-
High-dose dual therapy versus bismuth-containing quadruple therapy for the treatment of helicobacter pylori infection: A meta-analysis of randomized controlled trials.Saudi J Gastroenterol. 2023 Mar-Apr;29(2):88-94. doi: 10.4103/sjg.sjg_532_22. Saudi J Gastroenterol. 2023. PMID: 36960527 Free PMC article.
-
Efficacy and safety of bismuth-containing quadruple treatment and concomitant treatment for first-line Helicobacter pylori eradication: A systematic review and meta-analysis.Microb Pathog. 2021 Mar;152:104661. doi: 10.1016/j.micpath.2020.104661. Epub 2020 Nov 27. Microb Pathog. 2021. PMID: 33249167
-
Bismuth quadruple regimen with tetracycline or doxycycline versus three-in-one single capsule as third-line rescue therapy for Helicobacter pylori infection: Spanish data of the European Helicobacter pylori Registry (Hp-EuReg).Helicobacter. 2020 Oct;25(5):e12722. doi: 10.1111/hel.12722. Epub 2020 Jul 13. Helicobacter. 2020. PMID: 32656898
-
High dose dual therapy versus bismuth quadruple therapy for Helicobacter pylori eradication treatment: A systematic review and meta-analysis.Medicine (Baltimore). 2019 Feb;98(7):e14396. doi: 10.1097/MD.0000000000014396. Medicine (Baltimore). 2019. PMID: 30762742 Free PMC article.
Cited by
-
Current and Future Perspectives on the Management of Helicobacter pylori: A Narrative Review.Antibiotics (Basel). 2024 Jun 10;13(6):541. doi: 10.3390/antibiotics13060541. Antibiotics (Basel). 2024. PMID: 38927207 Free PMC article. Review.
-
Effect of an individualized bismuth quadruple regimen guided by 10-day or 14-day antibiotic susceptibility testing for first-line eradication treatment of Helicobacter pylori in Ningxia, China.Front Med (Lausanne). 2025 Jan 10;11:1510376. doi: 10.3389/fmed.2024.1510376. eCollection 2024. Front Med (Lausanne). 2025. PMID: 39867921 Free PMC article.
-
Overcoming antibiotic-resistant Helicobacter pylori infection: Current challenges and emerging approaches.World J Gastroenterol. 2025 Mar 14;31(10):102289. doi: 10.3748/wjg.v31.i10.102289. World J Gastroenterol. 2025. PMID: 40093672 Free PMC article. Review.
-
Empirical versus tailored therapy based on genotypic resistance detection for Helicobacter pylori eradication: a systematic review and meta-analysis.Therap Adv Gastroenterol. 2023 Aug 31;16:17562848231196357. doi: 10.1177/17562848231196357. eCollection 2023. Therap Adv Gastroenterol. 2023. PMID: 37667805 Free PMC article.
-
Efficacy and safety of triple therapy with vonoprazan for Helicobacter pylori eradication: A multicenter, prospective, randomized controlled trial.World J Gastroenterol. 2025 Jul 28;31(28):109001. doi: 10.3748/wjg.v31.i28.109001. World J Gastroenterol. 2025. PMID: 40741473 Free PMC article. Clinical Trial.
References
-
- Yang L, Kartsonaki C, Yao P, de Martel C, Plummer M, Chapman D, et al. . The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health. (2021) 6:e888–96. 10.1016/S2468-2667(21)00164-X - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

