Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter baumannii Infection
- PMID: 35402433
- PMCID: PMC8987773
- DOI: 10.3389/fmed.2022.793615
Convergence of Biofilm Formation and Antibiotic Resistance in Acinetobacter baumannii Infection
Abstract
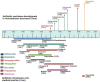
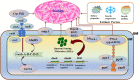
Acinetobacter baumannii (A. baumannii) is a leading cause of nosocomial infections as this pathogen has certain attributes that facilitate the subversion of natural defenses of the human body. A. baumannii acquires antibiotic resistance determinants easily and can thrive on both biotic and abiotic surfaces. Different resistance mechanisms or determinants, both transmissible and non-transmissible, have aided in this victory over antibiotics. In addition, the propensity to form biofilms (communities of organism attached to a surface) allows the organism to persist in hospitals on various medical surfaces (cardiac valves, artificial joints, catheters, endotracheal tubes, and ventilators) and also evade antibiotics simply by shielding the bacteria and increasing its ability to acquire foreign genetic material through lateral gene transfer. The biofilm formation rate in A. baumannii is higher than in other species. Recent research has shown how A. baumannii biofilm-forming capacity exerts its effect on resistance phenotypes, development of resistome, and dissemination of resistance genes within biofilms by conjugation or transformation, thereby making biofilm a hotspot for genetic exchange. Various genes control the formation of A. baumannii biofilms and a beneficial relationship between biofilm formation and "antimicrobial resistance" (AMR) exists in the organism. This review discusses these various attributes of the organism that act independently or synergistically to cause hospital infections. Evolution of AMR in A. baumannii, resistance mechanisms including both transmissible (hydrolyzing enzymes) and non-transmissible (efflux pumps and chromosomal mutations) are presented. Intrinsic factors [biofilm-associated protein, outer membrane protein A, chaperon-usher pilus, iron uptake mechanism, poly-β-(1, 6)-N-acetyl glucosamine, BfmS/BfmR two-component system, PER-1, quorum sensing] involved in biofilm production, extrinsic factors (surface property, growth temperature, growth medium) associated with the process, the impact of biofilms on high antimicrobial tolerance and regulation of the process, gene transfer within the biofilm, are elaborated. The infections associated with colonization of A. baumannii on medical devices are discussed. Each important device-related infection is dealt with and both adult and pediatric studies are separately mentioned. Furthermore, the strategies of preventing A. baumannii biofilms with antibiotic combinations, quorum sensing quenchers, natural products, efflux pump inhibitors, antimicrobial peptides, nanoparticles, and phage therapy are enumerated.
Keywords: Acinetobacter baumannii; adult; antimicrobial resistance; biofilm prevention; biofilm regulation; biofilm-associated infections; paediatric.
Copyright © 2022 Roy, Chowdhury, Mukhopadhyay, Dutta and Basu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- WHO . Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016–2017. Geneva: World Health Organization; (2017).
-
- Centers for Disease Control and Prevention . Antibiotic resistant threats in the United States 2019. (2019). Available online at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-re... (accessed January 15, 2020).
Publication types
LinkOut - more resources
Full Text Sources

