Genotype-Guided Use of P2Y12 Inhibitors: A Review of Current State of the Art
- PMID: 35402528
- PMCID: PMC8983962
- DOI: 10.3389/fcvm.2022.850028
Genotype-Guided Use of P2Y12 Inhibitors: A Review of Current State of the Art
Abstract
The pharmacodynamics of the purinergic receptor type Y, subtype 12 (P2Y12) inhibitors has evolved. Our understanding of the metabolism of P2Y12 inhibitors has revealed polymorphisms that impact drug metabolism and antiplatelet efficacy, leading to genetic testing guided therapy. In addition, assays of platelet function and biochemistry have provided insight into our understanding of the efficacy of "antiplatelet" therapy, identifying patients with high or low platelet reactivity on P2Y12 therapy. Despite the data, the implementation of these testing modalities has not gained mainstream adoption across hospital systems. Given differences in potency between the three clinically available P2Y12 inhibitors, the balance between thrombotic and bleeding complications must be carefully considered, especially for the large proportion of patients at higher risk for bleeding. Here we review the current data for genetic and functional testing, risk assessment strategies, and guidelines for P2Y12 inhibitors guided therapy.
Keywords: P2Y12; function; genotype; guided therapy; platelet.
Copyright © 2022 Al-abcha, Radwan, Blais, Mazzaferri, Boudoulas, Essa and Gumina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
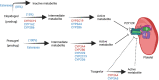

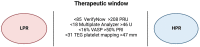

References
-
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. . 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2016) 134:e123–55. 10.1161/CIR.0000000000000453 - DOI - PubMed
-
- Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ, et al. . 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: a report of Journal of the American College of Cardiology. J Am Coll Cardiol. (2021) 77:629–58. 10.1016/j.jacc.2020.09.011 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

