Optimized Collagen Extraction Process to Obtain High Purity and Large Quantity of Collagen from Human Perirenal Adipose Tissue
- PMID: 35402618
- PMCID: PMC8989554
- DOI: 10.1155/2022/3628543
Optimized Collagen Extraction Process to Obtain High Purity and Large Quantity of Collagen from Human Perirenal Adipose Tissue
Abstract
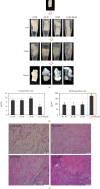
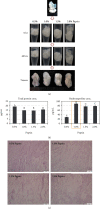
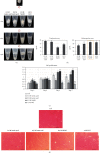
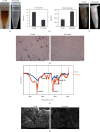
There is growing interest in human adipose tissue-derived collagen as a replacement for animal origin or synthetic materials. Large amounts of adipose tissues around the kidney are being discarded after kidney surgery; thus, we planned to use this tissue as a potentially ideal source of human collagen. Optimization of the collagen extraction process can contribute to the quality, quantity, supply, and cost of collagen production. To extract highly purified and concentrated collagen from human perirenal adipose tissue, we developed a novel extraction process that is superior to the conventional methods in terms of extraction yield, in vitro cytocompatibility, and physicochemical aspects. The sequence of the process and optimized conditions are as follows: (1) destaining with 0.5% H2O2 for 1 h at 4°C, (2) noncollagenous proteins elimination with 1.5 M NaOH for 24 h at 4°C, (3) atelocollagen preparation with 1.0% pepsin for 48 h at 25°C, and (4) collagen hydrolysis with 1.0 M NaOH for 10 min at 60°C. The final product showed significantly increased hydroxyproline (355.26 ± 18.71 pg/mL) and glycine (22.752 μg/mL) content than the conventional acetic acid hydrolyzed collagen (164.13 ± 1.11 pg/mL and 0.947 μg/mL, respectively). The lyophilized collagen showed more specific peaks for amides A, B, I, II, and III on FT-IR analysis and showed a further native architecture of collagen fibrils in scanning electron microscope images. Therefore, the optimized process can be an effective protocol for extracting collagen from human perirenal adipose tissue.
Copyright © 2022 Eun Hye Lee et al.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Nagai T., Suzuki N. Isolation of collagen from fish waste material—skin, bone and fins. Food Chemistry . 2000;68(3):277–281. doi: 10.1016/S0308-8146(99)00188-0. - DOI
-
- Helcke T. Gelatin, the food technologist’s friend or foe. International Food Ingredients. . 2000;1:6–8.
-
- Mori S., Kiuchi S., Ouchi A., Hase T., Murase T. Characteristic expression of extracellular matrix in subcutaneous adipose tissue development and adipogenesis; comparison with visceral adipose tissue. International Journal of Biological Sciences . 2014;10(8):825–833. doi: 10.7150/ijbs.8672. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

