Agonist and antagonist TRUPATH assays for G protein-coupled receptors
- PMID: 35403009
- PMCID: PMC8983427
- DOI: 10.1016/j.xpro.2022.101259
Agonist and antagonist TRUPATH assays for G protein-coupled receptors
Abstract
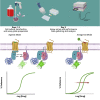
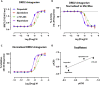
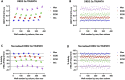
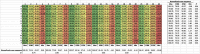
TRUPATH is a bioluminescence resonance energy transfer-based platform for quantifying G protein-coupled receptor activity via dissociation of heterotrimeric G protein biosensors. Here, we present protocols for agonist and antagonist TRUPATH assays in the 384-well plate format, thereby providing an opportunity for higher throughput. We also provide both data analysis and quality control analyses for these assays, along with considerations for assay optimization and solutions for troubleshooting needs that may be encountered. For complete details on the use and execution of this protocol, please refer to Olsen et al. (2020).
Keywords: Biotechnology and bioengineering; Cell Biology; Cell-based Assays; High Throughput Screening; Molecular Biology; Molecular/Chemical Probes; Signal Transduction.
© 2022 The Authors.
Conflict of interest statement
J.F.D., R.H.J.O., and B.L.R. are co-inventors of the TRUPATH platform for which the University of North Carolina at Chapel Hill has filed a patent.
Figures








References
-
- Chakraborty S., Uprety R., Daibani A.E., Rouzic V.L., Hunkele A., Appourchaux K., Eans S.O., Nuthikattu N., Jilakara R., Thammavong L., et al. Kratom alkaloids as probes for opioid receptor function: pharmacological characterization of minor indole and oxindole alkaloids from kratom. ACS Chem. Neurosci. 2021;12:2661–2678. - PMC - PubMed
-
- Chakraborty S., DiBerto J.F., Faouzi A., Bernhard S.M., Gutridge A.M., Ramsey S., Zhou Y., Provasi D., Nuthikattu N., Jilakara R., et al. A novel mitragynine analog with low-efficacy mu opioid receptor agonism displays antinociception with attenuated adverse effects. J. Med. Chem. 2021;64:13873–13892. - PMC - PubMed
-
- Du J., Wang D., Fan H., Xu C., Tai L., Lin S., Han S., Tan Q., Wang X., Xu T., et al. Structures of human mGlu2 and mGlu7 homo- and heterodimers. Nature. 2021;594:589–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

