Rat Spinal Cord Injury Associated with Spasticity Leads to Widespread Changes in the Regulation of Retained Introns
- PMID: 35403103
- PMCID: PMC8985541
- DOI: 10.1089/neur.2021.0042
Rat Spinal Cord Injury Associated with Spasticity Leads to Widespread Changes in the Regulation of Retained Introns
Abstract
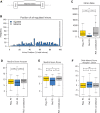
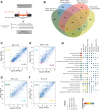
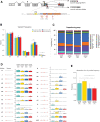
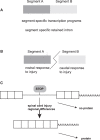
To determine molecular changes that correlate with long-term physiological changes after spinal cord injury associated with spasticity, we used a complete transection model with an injury at sacral spinal level S2, wherein tail spasms develop in rats weeks to months post-injury. Using Illumina and nanopore sequencing, we found that from 12,266 expressed genes roughly 11% (1,342) change expression levels in the rats with spasticity. The transcription factor PU.1 (Spi-1 proto-oncogene) and several of its known regulated genes were upregulated during injury, possibly reflecting changes in cellular composition. In contrast to widespread changes in gene expression, only a few changes in alternative exon usage could be detected because of injury. There were more than 1,000 changes in retained intron usage, however. Unexpectedly, most of these retained introns have not been described yet but could be validated using direct RNA nanopore sequencing. In addition to changes from injury, our model allowed regional analysis of gene expression. Comparing the segments rostral and caudal to the injury site in naïve animals showed 525 differentially regulated genes and differential regional use of retained introns. We did not detect changes in the serotonin receptor 2C editing that were implicated previously in this spinal cord injury model. Our data suggest that regulation of intron retention of polyadenylated pre-mRNA is an important regulatory mechanism in the spinal cord under both physiological and pathophysiological conditions.
Keywords: gene expression; mRNA; pre-mRNA splicing; spasticity.
© Samantha N. Hart et al., 2022; Published by Mary Ann Liebert, Inc.
Conflict of interest statement
No competing financial interests exist.
Figures


Similar articles
-
Global gene expression analysis of rodent motor neurons following spinal cord injury associates molecular mechanisms with development of postinjury spasticity.J Neurophysiol. 2010 Feb;103(2):761-78. doi: 10.1152/jn.00609.2009. Epub 2009 Nov 25. J Neurophysiol. 2010. PMID: 19939961
-
Serotonin 2C receptor alternative splicing in a spinal cord injury model.Neurosci Lett. 2013 Jan 4;532:49-54. doi: 10.1016/j.neulet.2012.10.034. Epub 2012 Nov 2. Neurosci Lett. 2013. PMID: 23123772
-
Changes in 5-HT1F receptor expression in rats with spasticity following spinal cord injury.Neurosci Lett. 2023 Jan 10;793:136988. doi: 10.1016/j.neulet.2022.136988. Epub 2022 Nov 26. Neurosci Lett. 2023. PMID: 36471527
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
-
Intron retention in viruses and cellular genes: Detention, border controls and passports.Wiley Interdiscip Rev RNA. 2018 May;9(3):e1470. doi: 10.1002/wrna.1470. Epub 2018 Mar 6. Wiley Interdiscip Rev RNA. 2018. PMID: 29508942 Free PMC article. Review.
Cited by
-
Alzheimer's disease pathogenetic progression is associated with changes in regulated retained introns and editing of circular RNAs.Front Mol Neurosci. 2023 May 5;16:1141079. doi: 10.3389/fnmol.2023.1141079. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37266374 Free PMC article.
References
-
- Skold, C., Levi, R., and Seiger, A. (1999). Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch. Phys. Med. Rehabil. 80, 1548–1557. - PubMed
-
- Finnerup, N.B. (2017). Neuropathic pain and spasticity: intricate consequences of spinal cord injury. Spinal Cord 55, 1046–1050. - PubMed
-
- Murray, K.C., Nakae, A., Stephens, M.J., Rank, M., D'Amico, J., Harvey, P.J., Li, X., Harris, R.L., Ballou, E.W., Anelli, R., Heckman, C.J., Mashimo, T., Vavrek, R., Sanelli, L., Gorassini, M.A., Bennett, D.J., and Fouad, K. (2010). Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 16, 694–700. - PMC - PubMed
-
- Di Narzo, A.F., Kozlenkov, A., Ge, Y., Zhang, B., Sanelli, L., May, Z., Li, Y., Fouad, K., Cardozo, C., Koonin, E.V., Bennett, D.J., and Dracheva, S. (2015). Decrease of mRNA editing after spinal cord injury is caused by down-regulation of ADAR2 that is triggered by inflammatory response. Sci. Rep. 5, 12615. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
