Bayesian sample size determination for diagnostic accuracy studies
- PMID: 35403239
- PMCID: PMC9325402
- DOI: 10.1002/sim.9393
Bayesian sample size determination for diagnostic accuracy studies
Abstract
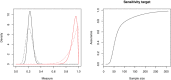
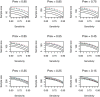
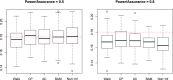
The development of a new diagnostic test ideally follows a sequence of stages which, among other aims, evaluate technical performance. This includes an analytical validity study, a diagnostic accuracy study, and an interventional clinical utility study. In this article, we propose a novel Bayesian approach to sample size determination for the diagnostic accuracy study, which takes advantage of information available from the analytical validity stage. We utilize assurance to calculate the required sample size based on the target width of a posterior probability interval and can choose to use or disregard the data from the analytical validity study when subsequently inferring measures of test accuracy. Sensitivity analyses are performed to assess the robustness of the proposed sample size to the choice of prior, and prior-data conflict is evaluated by comparing the data to the prior predictive distributions. We illustrate the proposed approach using a motivating real-life application involving a diagnostic test for ventilator associated pneumonia. Finally, we compare the properties of the approach against commonly used alternatives. The results show that, when suitable prior information is available, the assurance-based approach can reduce the required sample size when compared to alternative approaches.
Keywords: Bayesian assurance; binomial intervals; contingency tables; power calculations; sensitivity; specificity.
© 2022 The Authors. Statistics in Medicine published by John Wiley & Sons Ltd.
Figures

References
-
- Jiroutek MR, Muller KE, Kupper LL, Stewart PW. A new method for choosing sample size for confidence interval–Based inferences. Biometrics. 2003;59(3):580‐590. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
