Overall Vertical Transmission of Hepatitis C Virus, Transmission Net of Clearance, and Timing of Transmission
- PMID: 35403676
- PMCID: PMC9989130
- DOI: 10.1093/cid/ciac270
Overall Vertical Transmission of Hepatitis C Virus, Transmission Net of Clearance, and Timing of Transmission
Abstract
Background: It is widely accepted that the risk of hepatitis C virus (HCV) vertical transmission (VT) is 5%-6% in monoinfected women, and that 25%-40% of HCV infection clears spontaneously within 5 years. However, there is no consensus on how VT rates should be estimated, and there is a lack of information on VT rates "net" of clearance.
Methods: We reanalyzed data on 1749 children in 3 prospective cohorts to obtain coherent estimates of overall VT rate and VT rates net of clearance at different ages. Clearance rates were used to impute the proportion of uninfected children who had been infected and then cleared before testing negative. The proportion of transmission early in utero, late in utero, and at delivery was estimated from data on the proportion of HCV RNA positive within 3 days of birth, and differences between elective cesarean and nonelective cesarean deliveries.
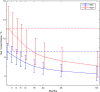
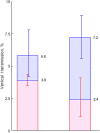
Results: Overall VT rates were 7.2% (95% credible interval [CrI], 5.6%-8.9%) in mothers who were human immunodeficiency virus (HIV) negative and 12.1% (95% CrI, 8.6%-16.8%) in HIV-coinfected women. The corresponding rates net of clearance at 5 years were 2.4% (95% CrI, 1.1%-4.1%), and 4.1% (95% CrI, 1.7%-7.3%). We estimated that 24.8% (95% CrI, 12.1%-40.8%) of infections occur early in utero, 66.0% (95% CrI, 42.5%-83.3%) later in utero, and 9.3% (95% CrI, 0.5%-30.6%) during delivery.
Conclusions: Overall VT rates are about 24% higher than previously assumed, but the risk of infection persisting beyond age 5 years is about 38% lower. The results can inform design of trials of interventions to prevent or treat pediatric HCV infection, and strategies to manage children exposed in utero.
Keywords: HCV; hepatitis C virus; net transmission; spontaneous clearance; vertical transmission.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C. T. has received honoraria for presentations from ViiV Healthcare (paid to author); reports grants or contracts unrelated to this work (paid to institution) from the European Commission, Child Health CIO, Public Health England, Penta Foundation, and ViiV Healthcare via Penta Foundation; and has participated on the Infectious Disease in Pregnancy Screening Programme Advisory Board. I. J. C. reports a Medical Research Council Global Health Trials development grant and a Gilead HCV Elimination competitive grant, both paid to institution and unrelated to this work. E. C. reports a contract or grant from ViiV Healthcare paid to institution via the Penta Foundation. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Comment in
-
Elimination Means Everyone: Targeting Hepatitis C in Infants and Pregnant Patients.Clin Infect Dis. 2023 Mar 4;76(5):920-922. doi: 10.1093/cid/ciac330. Clin Infect Dis. 2023. PMID: 35475916 No abstract available.
References
-
- World Health Organization . Combating hepatitis B and C to reach elimination by 2030. Geneva, Switzerland: WHO, 2016.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

