The effect of trastuzumab on cardiac function in patients with HER2-positive metastatic breast cancer and reduced baseline left ventricular ejection fraction
- PMID: 35403708
- PMCID: PMC9320802
- DOI: 10.1002/ijc.34024
The effect of trastuzumab on cardiac function in patients with HER2-positive metastatic breast cancer and reduced baseline left ventricular ejection fraction
Abstract
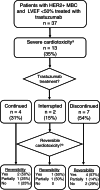
We investigated the effect of trastuzumab on cardiac function in a real-world historic cohort of patients with HER2-positive metastatic breast cancer (MBC) with reduced baseline left ventricular ejection fraction (LVEF). Thirty-seven patients with HER2-positive MBC and baseline LVEF of 40% to 49% were included. Median LVEF was 46% (interquartile range [IQR] 44%-48%) and median follow-up was 18 months (IQR 9-34 months). During this period, the LVEF did not worsen in 24/37 (65%) patients, while 13/37 (35%) patients developed severe cardiotoxicity defined as LVEF <40% with median time to severe cardiotoxicity of 7 months (IQR 4-10 months) after beginning trastuzumab. Severe cardiotoxicity was reversible (defined as LVEF increase to a value <5%-points below baseline value) in 7/13 (54%) patients, partly reversible (defined as absolute LVEF increase ≥10%-points from nadir to a value >5%-points below baseline) in 3/13 (23%) patients and irreversible (defined as absolute LVEF increase <10%-points from nadir and to a value >5%-points below baseline) in 3/13 (23%) patients. Likelihood of reversibility was numerically higher in patients who received cardio-protective medications (CPM), including ACE-inhibitors, beta-blockers and angiotensine-2 inhibitors, compared to those who did not receive any CPM (71% vs 13%, P = .091). Sixty-five percent of patients who received trastuzumab for HER2-positive MBC did not develop severe cardiotoxicity during a median follow-up of 18 months, despite having a compromised baseline LVEF. If severe cardiotoxicity occurred, it was at least partly reversible in more than two-thirds of the cases. Risks and benefits of trastuzumab use should be balanced carefully in this vulnerable population.
Keywords: HER2-positive metastatic breast cancer; cardiotoxicity; impaired baseline LVEF; trastuzumab.
© 2022 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
The authors declare no potential conflict of interests.
Figures
References
-
- Gullo G, Zuradelli M, Sclafani F, Santoro A, Crown J. Durable complete response following chemotherapy and trastuzumab for metastatic HER2‐positive breast cancer. Ann Oncol. 2012;23:2204‐2205. - PubMed
-
- Steenbruggen TG, Bouwer NI, Smorenburg CH, et al. Radiological complete remission in HER2‐positive metastatic breast cancer patients: what to do with trastuzumab? Breast Cancer Res Treat. 2019;178:597‐605. - PubMed
-
- Herceptin [package insert]. San Francisco, CA: Genentech, Inc.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

