Efficacy and safety of cotadutide, a dual glucagon-like peptide-1 and glucagon receptor agonist, in a randomized phase 2a study of patients with type 2 diabetes and chronic kidney disease
- PMID: 35403793
- PMCID: PMC9323481
- DOI: 10.1111/dom.14712
Efficacy and safety of cotadutide, a dual glucagon-like peptide-1 and glucagon receptor agonist, in a randomized phase 2a study of patients with type 2 diabetes and chronic kidney disease
Abstract
Aim: To assess the efficacy, safety and tolerability of cotadutide in patients with type 2 diabetes mellitus and chronic kidney disease.
Materials and methods: In this phase 2a study (NCT03550378), patients with body mass index 25-45 kg/m2 , estimated glomerular filtration rate 30-59 ml/min/1.73 m2 and type 2 diabetes [glycated haemoglobin 6.5-10.5% (48-91 mmol/mol)] controlled with insulin and/or oral therapy combination, were randomized 1:1 to once-daily subcutaneous cotadutide (50-300 μg) or placebo for 32 days. The primary endpoint was plasma glucose concentration assessed using a mixed-meal tolerance test.
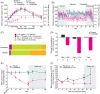
Results: Participants receiving cotadutide (n = 21) had significant reductions in the mixed-meal tolerance test area under the glucose concentration-time curve (-26.71% vs. +3.68%, p < .001), more time in target glucose range on continuous glucose monitoring (+14.79% vs. -21.23%, p = .001) and significant reductions in absolute bodyweight (-3.41 kg vs. -0.13 kg, p < .001) versus placebo (n = 20). In patients with baseline micro- or macroalbuminuria (n = 18), urinary albumin-to-creatinine ratios decreased by 51% at day 32 with cotadutide versus placebo (p = .0504). No statistically significant difference was observed in mean change in estimated glomerular filtration rate between treatments. Mild/moderate adverse events occurred in 71.4% of participants receiving cotadutide and 35.0% receiving placebo.
Conclusions: We established the efficacy of cotadutide in this patient population, with significantly improved postprandial glucose control and reduced bodyweight versus placebo. Reductions in urinary albumin-to-creatinine ratios suggest potential benefits of cotadutide on kidney function, supporting further evaluation in larger, longer-term clinical trials.
© 2022 AstraZeneca. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
VP, TH, YC, MPe, LH, PA and LJ are employees and shareholders of AstraZeneca. TH has stock or stock options with AstraZeneca, GW Pharma and Jazz Pharma. FG has received payment for lectures, presentations, speaker bureaus, manuscript writing or educational events from Eli Lilly and Novo Nordisk. LR has participated in advisory panels for Novo Nordisk and acted as a consultant for and is a member of the Association of Statutory Health Insurance Physicians. HH is a steering committee member and consultant for clinical trials sponsored by AstraZeneca, has received research grants from AstraZeneca for the present manuscript, is an advisor for AbbVie, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Eli Lilly, Janssen, Gilead, Merck, MundiPharma, Mitsubishi Tanabe, NovoNordisk, Travere Pharmaceuticals, and has received grants from AbbVie, Boehringer Ingelheim, Janssen, and NovoNordisk. RM has received research support from AstraZeneca, royalties or licenses from Elsevier, lecture fees from Sanofi Aventis and NovoNordisk, has participated on the Advisory Board for Sanofi Aventis, is a non‐executive member of NHS Tayside Health Board, and a panel member of MRC Population and Systems Medicine Board. HS, BW and MPo declare that they have no competing interests. Editorial support was provided by Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

