Feasibility and efficacy of combined pancreatic islet-lung transplantation in cystic fibrosis-related diabetes-PIM study: A multicenter phase 1-2 trial
- PMID: 35403818
- PMCID: PMC9540675
- DOI: 10.1111/ajt.17058
Feasibility and efficacy of combined pancreatic islet-lung transplantation in cystic fibrosis-related diabetes-PIM study: A multicenter phase 1-2 trial
Abstract
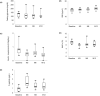
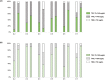
Cystic fibrosis-related diabetes (CFRD) is a common complication of cystic fibrosis (CF), and restoring metabolic control in these patients may improve their management after lung transplantation. In this multicenter, prospective, phase 1-2 trial, we evaluate the feasibility and metabolic efficacy of combined pancreatic islet-lung transplantation from a single donor in patients with CFRD, terminal respiratory failure, and poorly controlled diabetes. Islets were infused via the portal vein under local anesthesia, 1 week after lung transplantation. At 1 year, the primary outcome was transplant success as evaluated by a composite score including four parameters (weight, fasting glycemia, HbA1c, and insulin requirements). Ten participants (age: 24 years [17-31], diabetes duration: 8 years [4-12]) received a combined islet-lung transplant with 2892 IEQ/kg [2293-6185]. Transplant success was achieved in 7 out of 10 participants at 1-year post transplant. Fasting plasma C-peptide increased from 0.91 μg/L [0.56-1.29] to 1.15 μg/L [0.77-2.2], HbA1c decreased from 7.8% [6.5-8.3] (62 mmol/mol [48-67]) to 6.7% [5.5-8.0] (50 mmol/mol [37-64]), with 38% decrease in daily insulin doses. No complications related to the islet injection procedure were reported. In this pilot study, combined pancreatic islet-lung transplantation restored satisfactory metabolic control and pulmonary function in patients with CF, without increasing the morbidity of lung transplantation.
Trial registration: ClinicalTrials.gov NCT01548729.
Keywords: cystic fibrosis; islet transplantation; lung transplantation; respiratory failure; secondary diabetes.
© 2022 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures

References
-
- Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty‐sixth adult heart transplantation report ‐ 2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1056‐1066. doi:10.1016/j.healun.2019.08.004 - DOI - PMC - PubMed

