Genetic Deficiency of MicroRNA-15a/16-1 Confers Resistance to Neuropathological Damage and Cognitive Dysfunction in Experimental Vascular Cognitive Impairment and Dementia
- PMID: 35403823
- PMCID: PMC9189640
- DOI: 10.1002/advs.202104986
Genetic Deficiency of MicroRNA-15a/16-1 Confers Resistance to Neuropathological Damage and Cognitive Dysfunction in Experimental Vascular Cognitive Impairment and Dementia
Abstract
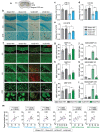
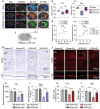
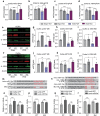
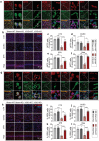
Chronic cerebral hypoperfusion-derived brain damage contributes to the progression of vascular cognitive impairment and dementia (VCID). Cumulative evidence has shown that microRNAs (miRs) are emerging as novel therapeutic targets for CNS disorders. In this study, it is sought to determine the regulatory role of miR-15a/16-1 in VCID. It is found that miR-15a/16-1 knockout (KO) mice exhibit less cognitive and sensorimotor deficits following VCID. Genetic deficiency of miR-15a/16-1 in VCID mice also mitigate myelin degeneration, axonal injury, and neuronal loss. Mechanistically, miR-15a/16-1 binds to the 3'-UTR of AKT3 and IL-10RA. Genetic deletion of miR-15a/16-1 increases AKT3 and IL-10RA expression in VCID brains, and intranasal delivery of AKT3 and IL-10RA siRNA-loaded nanoparticles partially reduce brain protection and cognitive recovery in miR-15a/16-1 KO mice after VCID. In conclusion, the miR-15a/16-1-IL/10RA/AKT3 axis plays a critical role in regulating vascular brain damage and cognitive decline after VCID. Targeting miR-15a/16-1 is a novel therapeutic approach for the treatment of VCID.
Keywords: AKT3; IL-10RA; grey matter lesions; miR-15a/16-1; microRNAs; vascular cognitive impairment and dementia; white matter lesions.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Khan A., Kalaria R. N., Corbett A., Ballard C., J. Geriatr. Psychiatry Neurol. 2016, 29, 281; - PubMed
- b) O'Brien J. T., Thomas A., Lancet 2015, 386, 1698; - PubMed
- c) O'Brien J. T., Erkinjuntti T., Reisberg B., Roman G., Sawada T., Pantoni L., Bowler J. V., Ballard C., DeCarli C., Gorelick P. B., Rockwood K., Burns A., Gauthier S., DeKosky S. T., Lancet Neurol. 2003, 2, 89. - PubMed
-
- a) Hase Y., Horsburgh K., Ihara M., Kalaria R. N., J. Neurochem. 2018, 144, 617; - PubMed
- b) Duncombe J., Kitamura A., Hase Y., Ihara M., Kalaria R. N., Horsburgh K., Clin. Sci. 2017, 131, 2451; - PubMed
- c) Mao L., Yang T., Li X., Lei X., Sun Y., Zhao Y., Zhang W., Gao Y., Sun B., Zhang F., J. Cereb. Blood Flow Metab. 2019, 39, 352. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
