Development of scaffold-free vascularized pancreatic beta-islets in vitro models by the anchoring of cell lines to a bioligand-functionalized gelatine substrate
- PMID: 35403934
- PMCID: PMC9001567
- DOI: 10.1007/s10856-022-06658-3
Development of scaffold-free vascularized pancreatic beta-islets in vitro models by the anchoring of cell lines to a bioligand-functionalized gelatine substrate
Abstract
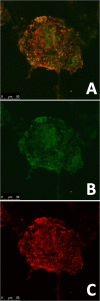
Bioengineered pancreatic β-islets have been widely advocated for the research and treatment of diabetes by offering both suitable cell culture models for the study of the pathology and the testing of new drugs and a therapy in those patients no longer responding to insulin administration and as an alternative to the shortage of donors for organ and islet transplantation. Unlike most of the studies published so far where pancreatic islets of pancreatic β-cells are encapsulated in hydrogels, this study demonstrate the formation of bioengineered pancreatic islets through cell anchoring to a gelatine-based biomaterial, PhenoDrive-Y, able to mimic the basement membrane of tissues. Through simple culture conditions, PhenoDrive-Y led human pancreatic β-cell lines and human umbilical endothelial cell lines to form organized structures closely resembling the natural vascularized pancreatic islets. When compared to gelatine, the cultures in presence of PhenoDrive-Y show higher degree of organization in tissue-like structures, a more pronounced endothelial sprouting and higher expression of typical cell markers. Noticeably, when challenged by hyperglycaemic conditions, the cells embedded in the PhenoDrive-Y assembled spheroids responded with higher levels of insulin production. In conclusion, the present work demonstrates the potential of PhenoDrive-Y as substrate for the development of bioengineered vascularized pancreatic islets and to be particularly suitable as a model for in vitro studies and testing of new therapeutics. Graphical abstract.
© 2022. The Author(s).
Conflict of interest statement
MS is the founder and Director of Tissue Click Ltd. PhenoDrive-Y was purchased from Tissue Click Ltd and the company neither provided any financial contribution to the work nor was involved in the experimental work, revision of the data and drafting of the paper that were all conducted independently by the authors.
Figures





Similar articles
-
Controlled Heterotypic Pseudo-Islet Assembly of Human β-Cells and Human Umbilical Vein Endothelial Cells Using Magnetic Levitation.Tissue Eng Part A. 2020 Apr;26(7-8):387-399. doi: 10.1089/ten.TEA.2019.0158. Epub 2019 Dec 20. Tissue Eng Part A. 2020. PMID: 31680653 Free PMC article.
-
Cellulose-based scaffolds enhance pseudoislets formation and functionality.Biofabrication. 2021 May 28;13(3). doi: 10.1088/1758-5090/ac00c3. Biofabrication. 2021. PMID: 34075893
-
Macroporous biohybrid cryogels for co-housing pancreatic islets with mesenchymal stromal cells.Acta Biomater. 2016 Oct 15;44:178-87. doi: 10.1016/j.actbio.2016.08.007. Epub 2016 Aug 6. Acta Biomater. 2016. PMID: 27506126
-
[Generating pancreatic islets organoids: Langerhanoids].Med Sci (Paris). 2022 Jan;38(1):52-58. doi: 10.1051/medsci/2021244. Epub 2022 Jan 21. Med Sci (Paris). 2022. PMID: 35060887 Review. French.
-
The pancreatic islet as a signaling hub.Adv Biol Regul. 2013 Jan;53(1):156-63. doi: 10.1016/j.jbior.2012.09.011. Epub 2012 Sep 28. Adv Biol Regul. 2013. PMID: 23073565 Review.
Cited by
-
New Frontiers in Three-Dimensional Culture Platforms to Improve Diabetes Research.Pharmaceutics. 2023 Feb 22;15(3):725. doi: 10.3390/pharmaceutics15030725. Pharmaceutics. 2023. PMID: 36986591 Free PMC article. Review.
-
Hydrogel-Based Vascularized Organ Tissue Engineering: A Systematized Review on Abdominal Organs.Gels. 2024 Oct 12;10(10):653. doi: 10.3390/gels10100653. Gels. 2024. PMID: 39451306 Free PMC article. Review.
-
Long-term cultures of human pancreatic islets in self-assembling peptides hydrogels.Front Bioeng Biotechnol. 2023 Feb 23;11:1105157. doi: 10.3389/fbioe.2023.1105157. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36911193 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

