Long-Term Treatment with Dimethyl Fumarate for Plaque Psoriasis in Routine Practice: Good Overall Effectiveness and Positive Effect on Impactful Areas
- PMID: 35403945
- PMCID: PMC8995418
- DOI: 10.1007/s13555-022-00714-0
Long-Term Treatment with Dimethyl Fumarate for Plaque Psoriasis in Routine Practice: Good Overall Effectiveness and Positive Effect on Impactful Areas
Abstract
Introduction: Dimethyl fumarate (DMF) is an oral compound to treat plaque psoriasis. Data on the treatment of patients with psoriasis affecting impactful areas are scarce. In this interim analysis of the prospective, noninterventional SKILL study, we summarized results of DMF treatment regarding effectiveness (overall and in impactful areas) and safety.
Methods: Data from 676 patients suffering from moderate-to-severe plaque psoriasis were analyzed after 52 weeks of DMF treatment. Of these, 257 had data available after 52 weeks. The considered impactful areas were nails, palms, soles, and scalp. Data analysis included observed cases (OC) and last observation carried forward (LOCF).
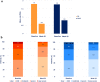
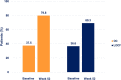
Results: All effectiveness parameters improved after 52 weeks. The Psoriasis Area and Severity Index score was reduced by 79.5% (OC) and 65.7% (LOCF). Compared with baseline, improvements were shown for 70.2% of the patients in their nail psoriasis [nail-Physician Global Assessment (PGA)] and for 57.3% in palmoplantar disease (palmoplantar-PGA). The proportion of patients with scalp-PGA 0/1 (clear/almost clear) increased significantly to 79.8% (OC) and 69.3% (LOCF, both p < 0.001) (versus 37.5% and 36.6% at baseline, respectively). Significant reduction of pruritus (p < 0.001) was also observed. No unexpected adverse drug reactions were observed.
Conclusion: Long-term treatment with DMF in routine practice showed good overall effectiveness and safety, and a positive effect on plaque-psoriasis-affected impactful areas.
Keywords: Dimethyl fumarate; Impactful areas; Nail; Palmoplantar; Psoriasis; Scalp.
© 2022. The Author(s).
Figures



References
-
- Reich K, Thaci D, Mrowietz U, Kamps A, Neureither M, Luger T. Efficacy and safety of fumaric acid esters in the long-term treatment of psoriasis—a retrospective study (FUTURE) JDDG J Dtsch Dermatol Ges. 2009;7:603–610. - PubMed
-
- Landeck L, Amasuno A, Pau-Charles I, Asadullah K. Identifying the active pharmaceutical ingredient from a mixture of fumaric acid esters for the treatment of psoriasis: hints from in vitro investigations. Adv Precis Med. 2017;2:325.
LinkOut - more resources
Full Text Sources

