Genome-Wide Investigation of Pasteurella multocida Identifies the Stringent Response as a Negative Regulator of Hyaluronic Acid Capsule Production
- PMID: 35404102
- PMCID: PMC9045168
- DOI: 10.1128/spectrum.00195-22
Genome-Wide Investigation of Pasteurella multocida Identifies the Stringent Response as a Negative Regulator of Hyaluronic Acid Capsule Production
Abstract
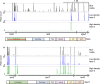
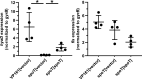
Pasteurella multocida is a Gram-negative capsulated bacterium responsible for a range of diseases that cause severe morbidity and mortality in livestock animals. The hyaluronic acid (HA) capsule produced by P. multocida serogroup A strains is a critical virulence factor. In this study, we utilized transposon-directed insertion site sequencing (TraDIS) to identify genes essential for in vitro growth of P. multocida and combined TraDIS with discontinuous density gradients (TraDISort) to identify genes required for HA capsule production and regulation in this pathogen. Analysis of mutants with a high cell density phenotype, indicative of the loss of extracellular capsule, led to the identification of 69 genes important for capsule production. These genes included all previously characterized genes in the capsule biosynthesis locus and fis and hfq, which encode known positive regulators of P. multocida capsule. Many of the other capsule-associated genes identified in this study were involved in regulation or activation of the stringent response, including spoT and relA, which encode proteins that regulate the concentration of guanosine alarmones. Disruption of the autoregulatory domains in the C-terminal half of SpoT using insertional mutagenesis resulted in reduced expression of capsule biosynthesis genes and an acapsular phenotype. Overall, these findings have greatly increased the understanding of hyaluronic acid capsule production and regulation in P. multocida. IMPORTANCE The bacterial pathogen P. multocida can cause serious disease in production animals, including fowl cholera in poultry, hemorrhagic septicemia in cattle and buffalo, atrophic rhinitis in pigs, and respiratory diseases in a range of livestock. P. multocida produces a capsule that is essential for systemic disease, but the complete mechanisms underlying synthesis and regulation of capsule production are not fully elucidated. A whole-genome analysis using TraDIS was undertaken to identify genes essential for growth in rich media and to obtain a comprehensive characterization of capsule production. Many of the capsule-associated genes identified in this study were involved in the stringent response to stress, a novel finding for this important animal pathogen.
Keywords: P. multocida; Pasteurella multocida; capsule; hyaluronic acid; stringent response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Woo YK, Kim JH. 2006. Fowl cholera outbreak in domestic poultry and epidemiological properties of Pasteurella multocida isolate. J Microbiol 44:344–353. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

