Alternative Splicing of the Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT) Is Regulated by RBFOX2 in Lymphoid Malignancies
- PMID: 35404107
- PMCID: PMC9119065
- DOI: 10.1128/mcb.00503-21
Alternative Splicing of the Aryl Hydrocarbon Receptor Nuclear Translocator (ARNT) Is Regulated by RBFOX2 in Lymphoid Malignancies
Abstract
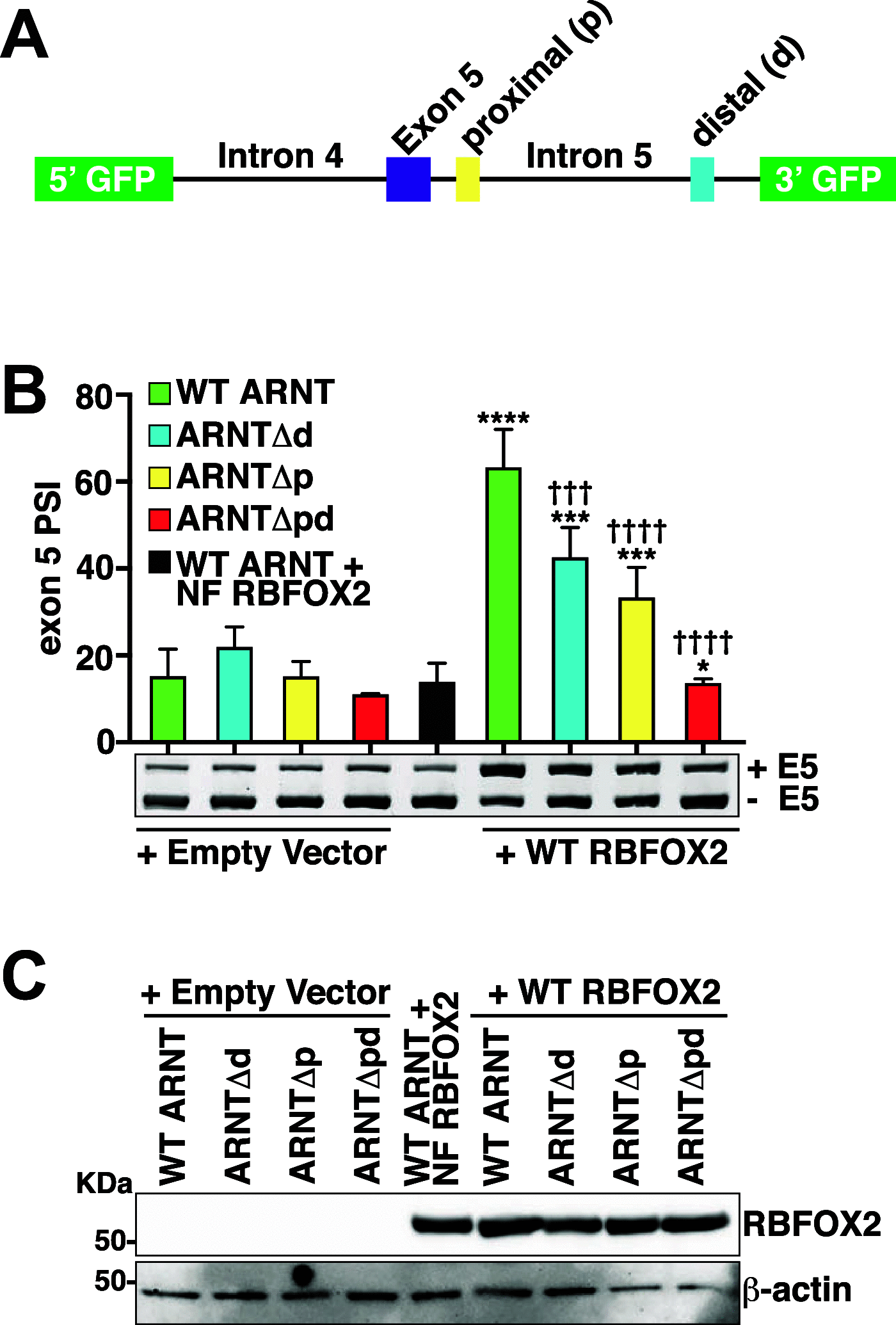
Aberrant alternative splicing (AS) of pre-mRNAs promotes the development and proliferation of cancerous cells. Accordingly, we had previously observed higher levels of the aryl hydrocarbon receptor nuclear translocator (ARNT) spliced variant isoform 1 in human lymphoid malignancies compared to that in normal lymphoid cells, which is a consequence of increased inclusion of alternative exon 5. ARNT is a transcription factor that has been implicated in the survival of various cancers. Notably, we found that ARNT isoform 1 promoted the growth and survival of lymphoid malignancies, but the regulatory mechanism controlling ARNT AS is unclear. Here, we report cis- and trans-regulatory elements which are important for the inclusion of ARNT exon 5. Specifically, we identified recognition motifs for the RNA-binding protein RBFOX2, which are required for RBFOX2-mediated exon 5 inclusion. RBFOX2 upregulation was observed in lymphoid malignancies, correlating with the observed increase in ARNT exon 5 inclusion. Moreover, suppression of RBFOX2 significantly reduced ARNT isoform 1 levels and cell growth. These observations reveal RBFOX2 as a critical regulator of ARNT AS in lymphoid malignancies and suggest that blocking the ARNT-specific RBFOX2 motifs to decrease ARNT isoform 1 levels is a viable option for targeting the growth of lymphoid malignancies.
Keywords: ARNT; RBFOX2; alternative splicing; lymphoid malignancies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
RNA splicing factor RBFOX2 is a key factor in the progression of cancer and cardiomyopathy.Clin Transl Med. 2024 Sep;14(9):e1788. doi: 10.1002/ctm2.1788. Clin Transl Med. 2024. PMID: 39243148 Free PMC article. Review.
-
Aryl hydrocarbon receptor nuclear translocator (ARNT) isoforms control lymphoid cancer cell proliferation through differentially regulating tumor suppressor p53 activity.Oncotarget. 2016 Mar 8;7(10):10710-22. doi: 10.18632/oncotarget.7539. Oncotarget. 2016. PMID: 26909609 Free PMC article.
-
AhR promotes phosphorylation of ARNT isoform 1 in human T cell malignancies as a switch for optimal AhR activity.Proc Natl Acad Sci U S A. 2022 Mar 22;119(12):e2114336119. doi: 10.1073/pnas.2114336119. Epub 2022 Mar 15. Proc Natl Acad Sci U S A. 2022. PMID: 35290121 Free PMC article.
-
Phosphorylation inhibits DNA-binding of alternatively spliced aryl hydrocarbon receptor nuclear translocator.Biochem Biophys Res Commun. 2005 Dec 9;338(1):660-7. doi: 10.1016/j.bbrc.2005.08.073. Epub 2005 Aug 19. Biochem Biophys Res Commun. 2005. PMID: 16129408
-
RBFOX2 protein domains and cellular activities.Biochem Soc Trans. 2014 Aug;42(4):1180-3. doi: 10.1042/BST20140050. Biochem Soc Trans. 2014. PMID: 25110022 Review.
Cited by
-
The evolution and structure/function of bHLH-PAS transcription factor family.Biochem Soc Trans. 2022 Jun 30;50(3):1227-1243. doi: 10.1042/BST20211225. Biochem Soc Trans. 2022. PMID: 35695677 Free PMC article. Review.
-
Targeting ARNT attenuates chemoresistance through destabilizing p38α-MAPK signaling in glioblastoma.Cell Death Dis. 2024 May 28;15(5):366. doi: 10.1038/s41419-024-06735-1. Cell Death Dis. 2024. PMID: 38806469 Free PMC article.
-
The complex biology of aryl hydrocarbon receptor activation in cancer and beyond.Biochem Pharmacol. 2023 Oct;216:115798. doi: 10.1016/j.bcp.2023.115798. Epub 2023 Sep 9. Biochem Pharmacol. 2023. PMID: 37696456 Free PMC article. Review.
-
Integrated pan-cancer analysis reveals the immunological and prognostic potential of RBFOX2 in human tumors.Front Pharmacol. 2024 May 31;15:1302134. doi: 10.3389/fphar.2024.1302134. eCollection 2024. Front Pharmacol. 2024. PMID: 38881877 Free PMC article.
-
RNA splicing factor RBFOX2 is a key factor in the progression of cancer and cardiomyopathy.Clin Transl Med. 2024 Sep;14(9):e1788. doi: 10.1002/ctm2.1788. Clin Transl Med. 2024. PMID: 39243148 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
