m6A RNA demethylase FTO promotes the growth, migration and invasion of pancreatic cancer cells through inhibiting TFPI-2
- PMID: 35404184
- PMCID: PMC9621031
- DOI: 10.1080/15592294.2022.2061117
m6A RNA demethylase FTO promotes the growth, migration and invasion of pancreatic cancer cells through inhibiting TFPI-2
Abstract
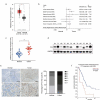
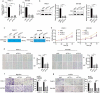
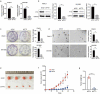
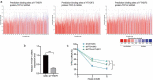
Pancreatic cancer (PC) is one of the most fatal cancers with a very poor prognosis. Here, we found that N6-methyladenosine (m6A) RNA demethylase fat mass and obesity-related protein (FTO) promote the growth, migration and invasion of PC. FTO expression level is increased in human PC and is associated with poor prognosis of PC patients. Knockdown of FTO increases m6A methylation of TFPI-2 mRNA in PC cells, thereby increasing mRNA stability via the m6A reader YTHDF1, resulting in up-regulation of TFPI-2 expression, and inhibits PC proliferation, colony formation, sphere formation, migration and invasion in vitro, as well as tumour growth in vivo. Rescue assay further confirms that FTO facilitates cancer progression by reducing the expression of TFPI-2. Mechanistically, FTO promotes the progression of PC at least partially through reducing m6A/YTHDF1 mediated TFPI-2 mRNA stability. Our findings reveal that FTO, as an m6A demethylase, plays a critical role in promoting PC growth, migration and invasion, suggesting that FTO may be a potential therapeutic target for treating PC.
Keywords: FTO; TFPI-2; m6A; pancreatic cancer; progression.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
