CPAP insufficiency leads to incomplete centrioles that duplicate but fragment
- PMID: 35404385
- PMCID: PMC9007748
- DOI: 10.1083/jcb.202108018
CPAP insufficiency leads to incomplete centrioles that duplicate but fragment
Abstract
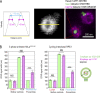
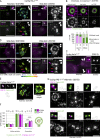
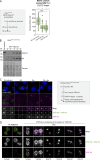
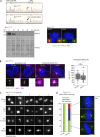
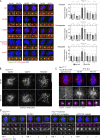
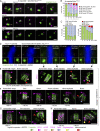
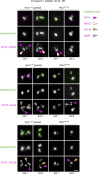
Centrioles are structures that assemble centrosomes. CPAP is critical for centrosome assembly, and its mutations are found in patients with diseases such as primary microcephaly. CPAP's centrosomal localization, its dynamics, and the consequences of its insufficiency in human cells are poorly understood. Here we use human cells genetically engineered for fast degradation of CPAP, in combination with superresolution microscopy, to address these uncertainties. We show that three independent centrosomal CPAP populations are dynamically regulated during the cell cycle. We confirm that CPAP is critical for assembly of human centrioles, but not for recruitment of pericentriolar material on already assembled centrioles. Further, we reveal that CPAP insufficiency leads to centrioles with incomplete microtubule triplets that can convert to centrosomes, duplicate, and form mitotic spindle poles, but fragment owing to loss of cohesion between microtubule blades. These findings further our basic understanding of the role of CPAP in centrosome biogenesis and help understand how CPAP aberrations can lead to human diseases.
© 2022 Vásquez Limeta et al.
Figures







Similar articles
-
Asymmetric spindle pole formation in CPAP-depleted mitotic cells.Biochem Biophys Res Commun. 2014 Feb 21;444(4):644-50. doi: 10.1016/j.bbrc.2014.01.129. Epub 2014 Feb 1. Biochem Biophys Res Commun. 2014. PMID: 24491538 Free PMC article.
-
Conserved TCP domain of Sas-4/CPAP is essential for pericentriolar material tethering during centrosome biogenesis.Proc Natl Acad Sci U S A. 2014 Jan 21;111(3):E354-63. doi: 10.1073/pnas.1317535111. Epub 2014 Jan 2. Proc Natl Acad Sci U S A. 2014. PMID: 24385583 Free PMC article.
-
Centrobin-centrosomal protein 4.1-associated protein (CPAP) interaction promotes CPAP localization to the centrioles during centriole duplication.J Biol Chem. 2014 May 30;289(22):15166-78. doi: 10.1074/jbc.M113.531152. Epub 2014 Apr 3. J Biol Chem. 2014. PMID: 24700465 Free PMC article.
-
Centriole inheritance.Prion. 2008 Jan-Mar;2(1):9-16. doi: 10.4161/pri.2.1.5064. Epub 2008 Jan 12. Prion. 2008. PMID: 19164929 Free PMC article. Review.
-
Centriolar satellites: busy orbits around the centrosome.Eur J Cell Biol. 2011 Dec;90(12):983-9. doi: 10.1016/j.ejcb.2011.07.007. Epub 2011 Sep 25. Eur J Cell Biol. 2011. PMID: 21945726 Review.
Cited by
-
9-fold symmetry is not essential for centriole elongation and formation of new centriole-like structures.Nat Commun. 2024 May 25;15(1):4467. doi: 10.1038/s41467-024-48831-y. Nat Commun. 2024. PMID: 38796459 Free PMC article.
-
Centrosomal organization of Cep152 provides flexibility in Plk4 and procentriole positioning.J Cell Biol. 2023 Dec 4;222(12):e202301092. doi: 10.1083/jcb.202301092. Epub 2023 Sep 14. J Cell Biol. 2023. PMID: 37707473 Free PMC article.
-
Predicting potential target genes in molecular biology experiments using machine learning and multifaceted data sources.iScience. 2024 Feb 23;27(3):109309. doi: 10.1016/j.isci.2024.109309. eCollection 2024 Mar 15. iScience. 2024. PMID: 38482491 Free PMC article.
-
Time-series reconstruction of the molecular architecture of human centriole assembly.Cell. 2024 Apr 25;187(9):2158-2174.e19. doi: 10.1016/j.cell.2024.03.025. Epub 2024 Apr 10. Cell. 2024. PMID: 38604175 Free PMC article.
-
Molecular basis promoting centriole triplet microtubule assembly.Nat Commun. 2024 Mar 22;15(1):2216. doi: 10.1038/s41467-024-46454-x. Nat Commun. 2024. PMID: 38519454 Free PMC article.
References
-
- Alieva, I.B., Zemskov E.A., Smurova K.M., Kaverina I.N., and Verin A.D.. 2013. The leading role of microtubules in endothelial barrier dysfunction: Disassembly of peripheral microtubules leaves behind the cytoskeletal reorganization. J. Cell. Biochem. 114:2258–2272. 10.1002/jcb.24575 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

