Sunvozertinib, a Selective EGFR Inhibitor for Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations
- PMID: 35404393
- PMCID: PMC9262839
- DOI: 10.1158/2159-8290.CD-21-1615
Sunvozertinib, a Selective EGFR Inhibitor for Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations
Abstract
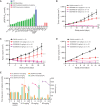
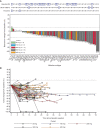
Epidermal growth factor receptor exon 20 insertion mutations (EGFRexon20ins) are detected in approximately 2% of patients with non-small cell lung cancer (NSCLC). Due to a lack of effective therapy, the prognosis of these patients is typically poor. Sunvozertinib (DZD9008) was designed as an oral, potent, irreversible, and selective EGFR tyrosine kinase inhibitor, showing activity against EGFRexon20ins and other mutations. In both cell lines and xenograft models, sunvozertinib shows potent antitumor activity. In the two ongoing phase I clinical studies, sunvozertinib was tolerated up to 400 mg once daily. The most common drug-related adverse events included diarrhea and skin rash. Antitumor efficacy was observed at the doses of 100 mg and above in patients with EGFRexon20ins NSCLC across different subtypes, with prior amivantamab treatment as well as with baseline brain metastasis. The median duration of response has not been reached.
Significance: We report the discovery and early clinical development of sunvozertinib, a potential treatment option for the unmet medical need of EGFRexon20ins NSCLC. This article is highlighted in the In This Issue feature, p. 1599.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
- doi: 10.1158/2159-8290.CD-12-7-ITI
References
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. . Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. - PubMed
-
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. . Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. - PubMed
-
- Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. . Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. - PubMed
-
- Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, et al. . Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res 1997;57:4838–48. - PubMed
-
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. . Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

