Evaluation of Long-term Outcomes Associated With Preterm Exposure to Antenatal Corticosteroids: A Systematic Review and Meta-analysis
- PMID: 35404395
- PMCID: PMC9002717
- DOI: 10.1001/jamapediatrics.2022.0483
Evaluation of Long-term Outcomes Associated With Preterm Exposure to Antenatal Corticosteroids: A Systematic Review and Meta-analysis
Abstract
Importance: Animal studies have found that antenatal corticosteroids affect many organs across multiple stages of life. However, the long-term outcomes in human children are not well understood.
Objective: To conduct a systematic review and meta-analysis of long-term outcomes associated with preterm exposure to antenatal corticosteroids compared with no exposure in all children as well as children with preterm and full-term birth.
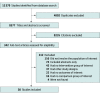
Data sources: Academic databases were searched for articles published from January 1, 2000, to October 29, 2021, including Ovid MEDLINE, Ovid Embase, PsycInfo, CINAHL (Cumulative Index of Nursing and Allied Health Literature), Web of Science, ClinicalTrials.gov, and Google Scholar. References of articles were also searched for relevant studies.
Study selection: Randomized clinical trials (RCTs), quasi-RCTs, and cohort studies that assessed long-term neurodevelopmental, psychological, or other outcomes at 1 year or older in those who had preterm exposure to antenatal corticosteroids were included. No language restrictions were set.
Data extraction and synthesis: Two reviewers independently extracted data using a piloted data extraction form. Data on study population, pregnancy characteristics, exposure to antenatal corticosteroids, and outcomes were collected. Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guidelines were followed, and random-effects models were used for the meta-analysis.
Main outcomes and measures: The primary outcome was an author-defined composite of any adverse neurodevelopmental and/or psychological disorder. The secondary outcomes included specific measures of psychological disorders; neurodevelopmental delay; and anthropometric, metabolic, and cardiorespiratory outcomes.
Results: A total of 30 studies met the inclusion criteria, and involved more than 1.25 million children who were at least 1 year of age when the outcomes were assessed. Exposure to a single course of antenatal corticosteroids for children with extremely preterm birth was associated with a significant reduction in risk of neurodevelopmental impairment (adjusted odds ratio, 0.69 [95% CI, 0.57-0.84]; I2 = 0%; low certainty). For children with late-preterm birth, exposure to antenatal corticosteroids was associated with a higher risk of investigation for neurocognitive disorders (n = 25 668 children; adjusted hazard ratio [aHR], 1.12 [95% CI, 1.05-1.20]; low certainty). For children with full-term birth, exposure to antenatal corticosteroids was associated with a higher risk of mental or behavioral disorders (n = 641 487 children; aHR, 1.47 [95% CI, 1.36-1.60]; low certainty) as well as proven or suspected neurocognitive disorders (n = 529 205 children; aHR, 1.16 [95% CI, 1.10-1.21]; low certainty).
Conclusions and relevance: Results of this study showed that exposure to a single course of antenatal corticosteroids was associated with a significantly lower risk of neurodevelopmental impairment in children with extremely preterm birth but a significantly higher risk of adverse neurocognitive and/or psychological outcomes in children with late-preterm and full-term birth, who made up approximately half of those with exposure to antenatal corticosteroids. The findings suggest a need for caution in administering antenatal corticosteroids.
Conflict of interest statement
Figures


Comment in
-
Use of Antenatal Corticosteroids for Risk of Preterm Birth-Is Timing Everything?JAMA Pediatr. 2022 Jun 1;176(6):e220480. doi: 10.1001/jamapediatrics.2022.0480. Epub 2022 Jun 6. JAMA Pediatr. 2022. PMID: 35404440 No abstract available.
-
Study Quality Must Be Considered When Evaluating Long-term Outcomes of Antenatal Corticosteroid Therapy in Children-Reply.JAMA Pediatr. 2022 Oct 1;176(10):1049. doi: 10.1001/jamapediatrics.2022.2858. JAMA Pediatr. 2022. PMID: 35969385 No abstract available.
-
Study Quality Must Be Considered When Evaluating Long-term Outcomes of Antenatal Corticosteroid Therapy in Children.JAMA Pediatr. 2022 Oct 1;176(10):1048-1049. doi: 10.1001/jamapediatrics.2022.2855. JAMA Pediatr. 2022. PMID: 35969393 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

