True seals achieved global distribution by breaking Bergmann's rule
- PMID: 35404473
- PMCID: PMC9321958
- DOI: 10.1111/evo.14488
True seals achieved global distribution by breaking Bergmann's rule
Abstract
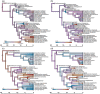
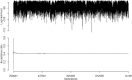
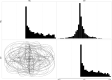
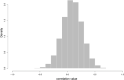
True seals (phocids) have achieved a global distribution by crossing the equator multiple times in their evolutionary history. This is remarkable, as warm tropical waters are regarded as a barrier to marine mammal dispersal and-following Bergmann's rule-may have limited crossings to small-bodied species only. Here, we show that ancestral phocids were medium sized and did not obviously follow Bergmann's rule. Instead, they ranged across a broad spectrum of environmental temperatures, without undergoing shifts in temperature- or size-related evolutionary rates following dispersals across the equator. We conclude that the tropics have not constrained phocid biogeography.
Keywords: Antitropical distribution; Bergmann's rule; Phocidae; biogeography; body size; sea surface temperature.
© 2022 The Authors. Evolution published by Wiley Periodicals LLC on behalf of The Society for the Study of Evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Adamczak, S.K. , Pabst, D.A. , McLellan, W.A. & Thorne, L.H. (2020) Do bigger bodies require bigger radiators? Insights into thermal ecology from closely related marine mammal species and implications for ecogeographic rules. J. Biogeogr., 47, 1193–1206.
-
- Amiot, R. , Göhlich, U.B. , Lecuyer, C. , De Muizon, C. , Cappetta, H. , Fourel, F. , Héran, M.‐A. & Martineau, F. (2008) Oxygen isotope compositions of phosphate from Middle Miocene–Early Pliocene marine vertebrates of Peru. Palaeogeogr. Palaeoclimatol. Palaeoecol., 264, 85–92.
-
- Amson, E. & Muizon, C.D. (2014) A new durophagous phocid (Mammalia: Carnivora) from the late Neogene of Peru and considerations on monachine seals phylogeny. Journal of Systematic Palaeontology, 12, 523‐548.
-
- Andersen, M. , Hjelset, A. , Gjertz, I. , Lydersen, C. & Gulliksen, B. (1999) Growth, age at sexual maturity and condition in bearded seals (Erignathus barbatus) from Svalbard, Norway. Polar Biol., 21, 179–185.
-
- Avery, G. & Klein, R.G. (2011) Review of fossil phocid and otariid seals from the southern and western coasts of South Africa. Trans. R. Soc. S. Afr., 66, 14–24.
